Excitatory neuron-specific suppression of the integrated stress response contributes to autism-related phenotypes in fragile X syndrome
- PMID: 37473758
- PMCID: PMC10592416
- DOI: 10.1016/j.neuron.2023.06.017
Excitatory neuron-specific suppression of the integrated stress response contributes to autism-related phenotypes in fragile X syndrome
Abstract
Dysregulation of protein synthesis is one of the key mechanisms underlying autism spectrum disorder (ASD). However, the role of a major pathway controlling protein synthesis, the integrated stress response (ISR), in ASD remains poorly understood. Here, we demonstrate that the main arm of the ISR, eIF2α phosphorylation (p-eIF2α), is suppressed in excitatory, but not inhibitory, neurons in a mouse model of fragile X syndrome (FXS; Fmr1-/y). We further show that the decrease in p-eIF2α is mediated via activation of mTORC1. Genetic reduction of p-eIF2α only in excitatory neurons is sufficient to increase general protein synthesis and cause autism-like behavior. In Fmr1-/y mice, restoration of p-eIF2α solely in excitatory neurons reverses elevated protein synthesis and rescues autism-related phenotypes. Thus, we reveal a previously unknown causal relationship between excitatory neuron-specific translational control via the ISR pathway, general protein synthesis, and core phenotypes reminiscent of autism in a mouse model of FXS.
Keywords: autism; fragile X syndrome; integrated stress response; mRNA translation.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.C.-M. is a member of Neuron’s advisory board and a shareholder of Altos Labs and Mikrovia.
Figures
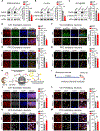

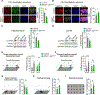



References
-
- Torossian A, Sare RM, Loutaev I, and Smith CB (2021). Increased rates of cerebral protein synthesis in Shank3 knockout mice: Implications for a link between synaptic protein deficit and dysregulated protein synthesis in autism spectrum disorder/intellectual disability. Neurobiol Dis 148, 105213. 10.1016/j.nbd.2020.105213. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

