Hepatitis Delta Infection: A Clinical Review
- PMID: 37473778
- PMCID: PMC10620035
- DOI: 10.1055/a-2133-8614
Hepatitis Delta Infection: A Clinical Review
Erratum in
-
Erratum to: Hepatitis Delta Infection: A Clinical Review.Semin Liver Dis. 2023 Aug;43(3):e1. doi: 10.1055/s-0043-1776037. Epub 2023 Oct 10. Semin Liver Dis. 2023. PMID: 37816394 Free PMC article. No abstract available.
Abstract
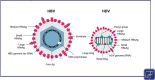
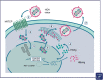
First discovered over 40 years ago, the hepatitis delta virus (HDV) is a unique RNA virus, requiring hepatitis B virus (HBV) antigens for its assembly, replication, and transmission. HBV and HDV can be acquired at the same time (coinfection) or HDV infection can occur in persons with chronic HBV (superinfection). Screening guidelines for HDV are inconsistent. While some guidelines recommend universal screening for all people with HBV, others recommend risk-based screening. Estimates of the global HDV prevalence range from 4.5 to 14.6% among persons with HBV; thus, there may be up to 72 million individuals with HDV worldwide. HDV is the most severe form of viral hepatitis. Compared to HBV monoinfection, HDV coinfection increases the risk of cirrhosis, hepatocellular carcinoma, hepatic decompensation, mortality, and necessity for liver transplant. Despite the severity of HDV, there are few treatment options. Pegylated interferon (off-label use) has long been the only available treatment, although bulevirtide is conditionally approved in some European countries. There are many potential treatments in development, but as yet, there are few effective and safe therapies for HDV infection. In conclusion, given the severity of HDV disease and the paucity of treatments, there is a great unmet need for HDV therapies.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
BP is part of the speakers' bureau for Gilead Sciences, Inc., a manufacturer of an investigational drug for hepatitis delta.
Figures


References
-
- He L F, Ford E, Purcell R H, London W T, Phillips J, Gerin J L. The size of the hepatitis delta agent. J Med Virol. 1989;27(01):31–33. - PubMed
-
- Lempp F A, Ni Y, Urban S. Hepatitis delta virus: insights into a peculiar pathogen and novel treatment options. Nat Rev Gastroenterol Hepatol. 2016;13(10):580–589. - PubMed
-
- Lucifora J, Delphin M. Current knowledge on hepatitis delta virus replication. Antiviral Res. 2020;179:104812. - PubMed

