A conserved arginine within the αC-helix of Erk1/2 is a latch of autoactivation and of oncogenic capabilities
- PMID: 37474104
- PMCID: PMC10458722
- DOI: 10.1016/j.jbc.2023.105072
A conserved arginine within the αC-helix of Erk1/2 is a latch of autoactivation and of oncogenic capabilities
Abstract
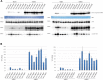
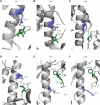
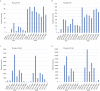
Eukaryotic protein kinases (EPKs) adopt an active conformation following phosphorylation of a particular activation loop residue. Most EPKs spontaneously autophosphorylate this residue. While structure-function relationships of the active conformation are essentially understood, those of the "prone-to-autophosphorylate" conformation are unclear. Here, we propose that a site within the αC-helix of EPKs, occupied by Arg in the mitogen-activated protein kinase (MAPK) Erk1/2 (Arg84/65), impacts spontaneous autophosphorylation. MAPKs lack spontaneous autoactivation, but we found that converting Arg84/65 of Erk1/2 to various residues enables spontaneous autophosphorylation. Furthermore, Erk1 molecules mutated in Arg84 are oncogenic. Arg84/65 thus obstructs the adoption of the "prone-to-autophosphorylate" conformation. All MAPKs harbor an Arg that is equivalent to Arg84/65 of Erks, whereas Arg is rarely found at the equivalent position in other EPKs. We observed that Arg84/65 of Erk1/2 interacts with the DFG motif, suggesting that autophosphorylation may be inhibited by the Arg84/65-DFG interactions. Erk1/2s mutated in Arg84/65 autophosphorylate not only the TEY motif, known as critical for catalysis, but also on Thr207/188. Our MS/MS analysis revealed that a large proportion of the Erk2R65H population is phosphorylated on Thr188 or on Tyr185 + Thr188, and a small fraction is phosphorylated on the TEY motif. No molecules phosphorylated on Thr183 + Thr188 were detected. Thus, phosphorylation of Thr183 and Thr188 is mutually exclusive suggesting that not only TEY-phosphorylated molecules are active but perhaps also those phosphorylated on Tyr185 + Thr188. The effect of mutating Arg84/65 may mimic a physiological scenario in which allosteric effectors cause Erk1/2 activation by autophosphorylation.
Keywords: ERK; MAP kinase; activation loop; active variants; autophosphorylation; eukaryotic protein kinases.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



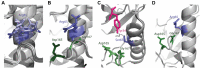






References
-
- Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Hanks S.K., Hunter T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification 1. FASEB J. 1995;9:576–596. - PubMed
-
- Beenstock J., Mooshayef N., Engelberg D. How do protein kinases take a selfie (autophosphorylate)? Trends Biochem. Sci. 2016;41:938–953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

