Alanyl-glutamine supplementation for Clostridioides difficile infection treatment (ACT): a double-blind randomised controlled trial study protocol
- PMID: 37474181
- PMCID: PMC10357635
- DOI: 10.1136/bmjopen-2023-075721
Alanyl-glutamine supplementation for Clostridioides difficile infection treatment (ACT): a double-blind randomised controlled trial study protocol
Abstract
Introduction: Clostridioides difficile is the leading cause of healthcare-associated infections in the USA, with an estimated 1 billion dollars in excess cost to the healthcare system annually. C. difficile infection (CDI) has high recurrence rate, up to 25% after first episode and up to 60% for succeeding episodes. Preliminary in vitro and in vivo studies indicate that alanyl-glutamine (AQ) may be beneficial in treating CDI by its effect on restoring intestinal integrity in the epithelial barrier, ameliorating inflammation and decreasing relapse.
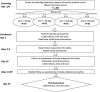
Methods and analysis: This study is a randomised, placebo-controlled, double-blind, phase II clinical trial. The trial is designed to determine optimal dose and safety of oral AQ at 4, 24 and 44 g doses administered daily for 10 days concurrent with standard treatment of non-severe or severe uncomplicated CDI in persons age 18 and older. The primary outcome of interest is CDI recurrence during 60 days post-treatment follow-up, with the secondary outcome of mortality during 60 days post-treatment follow-up. Exploratory analysis will be done to determine the impact of AQ supplementation on intestinal and systemic inflammation, as well as intestinal microbial and metabolic profiles.
Ethics and dissemination: The study has received University of Virginia Institutional Review Board approval (HSR200046, Protocol v9, April 2023). Findings will be disseminated via conference presentations, lectures and peer-reviewed publications.
Trial registration number: NCT04305769.
Keywords: clinical trials; gastroenterology; gastrointestinal infections; infectious diseases; randomized controlled trial.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CW is a medical advisor for Seres-109 of Aimmune and Seres Therapeutics and site PI for Rebyota Prospective Registry. The rest of the authors have no conflict of interest to disclose.
Figures
References
-
- Centers for Disease Control and Prevention (US) . Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (US), 2019. 10.15620/cdc:82532 - DOI
-
- McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and society for Healthcare epidemiology of America (SHEA). Clin Infect Dis 2018;66:e1–48. 10.1093/cid/cix1085 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous