Atypical Chemokine Receptor 3 "Senses" CXC Chemokine Receptor 4 Activation Through GPCR Kinase Phosphorylation
- PMID: 37474305
- PMCID: PMC11033958
- DOI: 10.1124/molpharm.123.000710
Atypical Chemokine Receptor 3 "Senses" CXC Chemokine Receptor 4 Activation Through GPCR Kinase Phosphorylation
Abstract
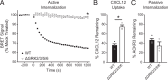
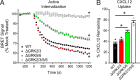
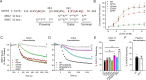
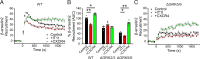
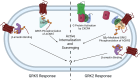
Atypical chemokine receptor 3 (ACKR3) is an arrestin-biased receptor that regulates extracellular chemokine levels through scavenging. The scavenging process restricts the availability of the chemokine agonist CXCL12 for the G protein-coupled receptor (GPCR) CXCR4 and requires phosphorylation of the ACKR3 C-terminus by GPCR kinases (GRKs). ACKR3 is phosphorylated by GRK2 and GRK5, but the mechanisms by which these kinases regulate the receptor are unresolved. Here we determined that GRK5 phosphorylation of ACKR3 results in more efficient chemokine scavenging and β-arrestin recruitment than phosphorylation by GRK2 in HEK293 cells. However, co-activation of CXCR4-enhanced ACKR3 phosphorylation by GRK2 through the liberation of Gβγ, an accessory protein required for efficient GRK2 activity. The results suggest that ACKR3 "senses" CXCR4 activation through a GRK2-dependent crosstalk mechanism, which enables CXCR4 to influence the efficiency of CXCL12 scavenging and β-arrestin recruitment to ACKR3. Surprisingly, we also found that despite the requirement for phosphorylation and the fact that most ligands promote β-arrestin recruitment, β-arrestins are dispensable for ACKR3 internalization and scavenging, suggesting a yet-to-be-determined function for these adapter proteins. Since ACKR3 is also a receptor for CXCL11 and opioid peptides, these data suggest that such crosstalk may also be operative in cells with CXCR3 and opioid receptor co-expression. Additionally, kinase-mediated receptor cross-regulation may be relevant to other atypical and G protein-coupled receptors that share common ligands. SIGNIFICANCE STATEMENT: The atypical receptor ACKR3 indirectly regulates CXCR4-mediated cell migration by scavenging their shared agonist CXCL12. Here, we show that scavenging and β-arrestin recruitment by ACKR3 are primarily dependent on phosphorylation by GRK5. However, we also show that CXCR4 co-activation enhances the contribution of GRK2 by liberating Gβγ. This phosphorylation crosstalk may represent a common feedback mechanism between atypical and G protein-coupled receptors with shared ligands for regulating the efficiency of scavenging or other atypical receptor functions.
Copyright © 2023 by The Author(s).
Figures





Update of
-
Atypical Chemokine Receptor 3 'Senses' CXC Chemokine Receptor 4 Activation Through GPCR Kinase Phosphorylation.bioRxiv [Preprint]. 2023 Mar 10:2023.02.25.530029. doi: 10.1101/2023.02.25.530029. bioRxiv. 2023. Update in: Mol Pharmacol. 2023 Oct;104(4):174-186. doi: 10.1124/molpharm.123.000710. PMID: 36865154 Free PMC article. Updated. Preprint.
References
-
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F (2005) The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280:35760–35766. - PubMed
-
- Bhandari D, Trejo J, Benovic JL, Marchese A (2007) Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem 282:36971–36979. - PubMed
-
- Boekhoff I, Inglese J, Schleicher S, Koch WJ, Lefkowitz RJ, Breer H (1994) Olfactory desensitization requires membrane targeting of receptor kinase mediated by beta gamma-subunits of heterotrimeric G proteins. J Biol Chem 269:37–40. - PubMed

