Efficient high-precision homology-directed repair-dependent genome editing by HDRobust
- PMID: 37474806
- PMCID: PMC10482697
- DOI: 10.1038/s41592-023-01949-1
Efficient high-precision homology-directed repair-dependent genome editing by HDRobust
Abstract
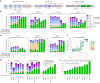
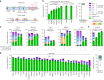
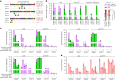
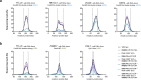
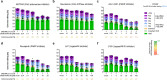
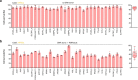
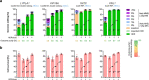
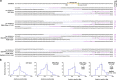
Homology-directed repair (HDR), a method for repair of DNA double-stranded breaks can be leveraged for the precise introduction of mutations supplied by synthetic DNA donors, but remains limited by low efficiency and off-target effects. In this study, we report HDRobust, a high-precision method that, via the combined transient inhibition of nonhomologous end joining and microhomology-mediated end joining, resulted in the induction of point mutations by HDR in up to 93% (median 60%, s.e.m. 3) of chromosomes in populations of cells. We found that, using this method, insertions, deletions and rearrangements at the target site, as well as unintended changes at other genomic sites, were largely abolished. We validated this approach for 58 different target sites and showed that it allows efficient correction of pathogenic mutations in cells derived from patients suffering from anemia, sickle cell disease and thrombophilia.
© 2023. The Author(s).
Conflict of interest statement
Related patent applications on repair gene modified cell lines (patent applicant: Max Planck Society; inventors: S.R. and T.M.; application number: EP17203591.7 and PCT/EP2018/059173; publication: 2018-10-17 WO2018189186; status: pending) and compounds for transient HDR increase (patent applicant: Max Planck Society; inventors: S.R. and T.M.; application number: EP18215071.4; publication: 2020-06-25 WO2020127738A1; status: pending) have been filed. All other authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

