Alterations in trimethylamine-N-oxide in response to Empagliflozin therapy: a secondary analysis of the EMMY trial
- PMID: 37475009
- PMCID: PMC10357596
- DOI: 10.1186/s12933-023-01920-6
Alterations in trimethylamine-N-oxide in response to Empagliflozin therapy: a secondary analysis of the EMMY trial
Abstract
Introduction: The relationship between sodium glucose co-transporter 2 inhibitors (SGLT2i) and trimethylamine N-oxide (TMAO) following acute myocardial infarction (AMI) is not yet explored.
Methods: In this secondary analysis of the EMMY trial (ClinicalTrials.gov registration: NCT03087773), changes in serum TMAO levels were investigated in response to 26-week Empagliflozin treatment following an AMI compared to the standard post-MI treatment. Additionally, the association of TMAO changes with clinical risk factors and cardiorenal biomarkers was assessed.
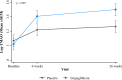
Results: The mean age of patients (N = 367) was 57 ± 9 years, 82% were males, and 14% had type 2 diabetes. In the Empagliflozin group, the median TMAO value was 2.62 µmol/L (IQR: 1.81) at baseline, 3.74 µmol/L (2.81) at 6 weeks, and 4.20 µmol/L (3.14) at 26 weeks. In the placebo group, the median TMAO value was 2.90 µmol/L (2.17) at baseline, 3.23 µmol/L (1.90) at 6 weeks, and 3.35 µmol/L (2.50) at 26 weeks. The serum TMAO levels increased significantly from baseline to week 6 (coefficient: 0.233; 95% confidence interval 0.149-0.317, p < 0.001) and week 26 (0.320, 0.236-0.405, p < 0.001). The average increase in TMAO levels over time (pinteraction = 0.007) was significantly higher in the Empagliflozin compared to the Placebo group. Age was positively associated with TMAO, whereas eGFR and LVEF were negatively associated with TMAO.
Conclusions: Our results are contrary to existing experimental studies that showed the positive impact of SGLT2i on TMAO precursors and cardiovascular events. Therefore, we recommend further research investigating the impact of SGLT2i therapy on acute and long-term changes in TMAO in cardiovascular cohorts.
Keywords: Clinical trial; Empagliflozin; Heart failure; Myocardial infarction; RCT; Trimethylamine N-oxide.
© 2023. The Author(s).
Conflict of interest statement
HS is on the advisory board and speakers bureau of Boehringer Ingelheim, NovoNordisk, Sanofi-Aventis, Amgen, AstraZeneca, Bayer, Eli Lilly, Kapsch, MSD, and Daiichi Sankyo. DvL is on the advisory board and speaker’s bureau of by Boehringer Ingelheim, Novartis, Sanova, Sanofi, Orion, AstraZeneca, Bayer Recardio, Vaxxinity and Daiichi Sankyo. FA reports no conflict of interest related to this study. All other authors report no conflict of interest related to this study.
Figures
Similar articles
-
Ketone body levels and its associations with cardiac markers following an acute myocardial infarction: a post hoc analysis of the EMMY trial.Cardiovasc Diabetol. 2024 Apr 27;23(1):145. doi: 10.1186/s12933-024-02221-2. Cardiovasc Diabetol. 2024. PMID: 38678253 Free PMC article. Clinical Trial.
-
Impact of the SGLT2-inhibitor empagliflozin on inflammatory biomarkers after acute myocardial infarction - a post-hoc analysis of the EMMY trial.Cardiovasc Diabetol. 2023 Jul 5;22(1):166. doi: 10.1186/s12933-023-01904-6. Cardiovasc Diabetol. 2023. PMID: 37407956 Free PMC article. Clinical Trial.
-
Effects of empagliflozin in women and men with acute myocardial infarction: An analysis from the EMMY trial.Hellenic J Cardiol. 2024 Jan-Feb;75:3-8. doi: 10.1016/j.hjc.2023.05.007. Epub 2023 May 24. Hellenic J Cardiol. 2024. PMID: 37236318 Clinical Trial.
-
Effects of empagliflozin on first and recurrent clinical events in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a secondary analysis of the EMPA-REG OUTCOME trial.Lancet Diabetes Endocrinol. 2020 Dec;8(12):949-959. doi: 10.1016/S2213-8587(20)30344-2. Lancet Diabetes Endocrinol. 2020. PMID: 33217335 Clinical Trial.
-
Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial.Cardiovasc Diabetol. 2020 Sep 25;19(1):148. doi: 10.1186/s12933-020-01127-z. Cardiovasc Diabetol. 2020. PMID: 32977831 Free PMC article. Clinical Trial.
Cited by
-
Microbiome-Derived Trimethylamine N-Oxide (TMAO) as a Multifaceted Biomarker in Cardiovascular Disease: Challenges and Opportunities.Int J Mol Sci. 2024 Nov 21;25(23):12511. doi: 10.3390/ijms252312511. Int J Mol Sci. 2024. PMID: 39684223 Free PMC article. Review.
-
The influence of acute and chronic coronary syndrome on the gut microbiome and downstream microbiome-derived metabolites-Microbiome in acute myocardial infarction-MIAMI-Trial.Basic Res Cardiol. 2025 Aug 13. doi: 10.1007/s00395-025-01134-9. Online ahead of print. Basic Res Cardiol. 2025. PMID: 40804540
-
Timing of SGLT2i initiation after acute myocardial infarction.Cardiovasc Diabetol. 2023 Sep 30;22(1):269. doi: 10.1186/s12933-023-02000-5. Cardiovasc Diabetol. 2023. PMID: 37777743 Free PMC article.
-
TMAO and diabetes: from the gut feeling to the heart of the problem.Nutr Diabetes. 2025 May 20;15(1):21. doi: 10.1038/s41387-025-00377-8. Nutr Diabetes. 2025. PMID: 40393987 Free PMC article. Review.
-
Urea, TMAO, betaine and other osmolytes as endogenous diuretics in heart failure and hypertension.Heart Fail Rev. 2025 Sep;30(5):1061-1074. doi: 10.1007/s10741-025-10530-1. Epub 2025 Jun 2. Heart Fail Rev. 2025. PMID: 40455373 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous