Meningococcal ACWY conjugate vaccine immunogenicity in adolescents with primary or secondary immune deficiencies, a prospective observational cohort study
- PMID: 37475057
- PMCID: PMC10360259
- DOI: 10.1186/s12969-023-00846-3
Meningococcal ACWY conjugate vaccine immunogenicity in adolescents with primary or secondary immune deficiencies, a prospective observational cohort study
Abstract
Background: Immunization with meningococcal ACWY conjugate vaccine induces protective antibodies against invasive meningococcal disease (IMD) caused by serogroups A, C, W and Y. We studied MenACWY-TT vaccine immunogenicity in adolescents with a heterogenous group of primary and secondary immune deficiency including patients with systemic lupus erythematosus, mixed connective tissue disease, vasculitis, uveitis, 22Q11 syndrome, sickle cell disease, and patients who underwent stem cell transplantation for bone marrow failure.
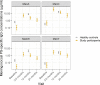
Findings: We enrolled 69 individuals aged 14-18 years diagnosed with a primary or secondary immune deficiency in a prospective observational cohort study. All patients received a single dose of MenACWY-TT vaccine during the catch-up campaign 2018-19 because of the IMD-W outbreak in the Netherlands. Capsular polysaccharide-specific (PS) IgG concentrations against MenACWY were measured before and 3-6, 12, and 24 months after vaccination. Overall, geometric mean concentrations (GMCs) of MenACWY-PS-specific IgG were lower in patients compared to data from healthy, aged-matched controls (n = 75) reaching significance at 12 months postvaccination for serogroup A and W (adjusted GMC ratios 0.26 [95% CI: 0.15-0.47] and 0.22 [95% CI: 0.10-0.49], respectively). No serious adverse events were reported by study participants.
Conclusions: The MenACWY conjugate vaccine was less immunogenic in adolescent patients with primary or secondary immunodeficiency compared to healthy controls, urging the need for further surveillance of these patients and supporting considerations for booster MenACWY conjugate vaccinations in these patient groups.
Keywords: Adolescents; Antibody responses; Autoimmune disease; Immunocompromised; Immunodeficiency; Inflammatory disease; MenACWY conjugate vaccination.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Meningococcal ACWY conjugate vaccine immunogenicity and safety in adolescents with juvenile idiopathic arthritis and inflammatory bowel disease: A prospective observational cohort study.Vaccine. 2023 Jun 7;41(25):3782-3789. doi: 10.1016/j.vaccine.2023.04.056. Epub 2023 May 15. Vaccine. 2023. PMID: 37198018
-
Safety and immunogenicity of a booster dose of meningococcal (groups A, C, W, and Y) polysaccharide diphtheria toxoid conjugate vaccine.Vaccine. 2016 Oct 17;34(44):5273-5278. doi: 10.1016/j.vaccine.2016.09.003. Epub 2016 Sep 15. Vaccine. 2016. PMID: 27642132 Clinical Trial.
-
Long-term immunogenicity and safety after a single dose of the quadrivalent meningococcal serogroups A, C, W, and Y tetanus toxoid conjugate vaccine in adolescents and adults: 5-year follow-up of an open, randomized trial.BMC Infect Dis. 2015 Oct 6;15:409. doi: 10.1186/s12879-015-1138-y. BMC Infect Dis. 2015. PMID: 26437712 Free PMC article. Clinical Trial.
-
Persistence of bactericidal antibodies following primary and booster MenACWY-TT vaccination of toddlers: A review of clinical studies.Vaccine. 2020 Jun 2;38(27):4236-4245. doi: 10.1016/j.vaccine.2020.02.048. Epub 2020 May 7. Vaccine. 2020. PMID: 32389497 Review.
-
Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence.Future Microbiol. 2019 May;14:563-580. doi: 10.2217/fmb-2018-0343. Epub 2019 May 16. Future Microbiol. 2019. PMID: 31091978 Review.
References
-
- Jansen MH, Rondaan C, Legger G, Minden K, Uziel Y, Toplak N et al. Efficacy, immunogenicity and safety of Vaccination in Pediatric Patients with Autoimmune Inflammatory Rheumatic Diseases (pedAIIRD): a systematic literature review for the 2021 update of the EULAR/PRES recommendations. Front Pead. 2022;10. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

