Mpox vaccine and infection-driven human immune signatures: an immunological analysis of an observational study
- PMID: 37475115
- PMCID: PMC10826035
- DOI: 10.1016/S1473-3099(23)00352-3
Mpox vaccine and infection-driven human immune signatures: an immunological analysis of an observational study
Abstract
Background: Monkeypox virus has recently infected more than 88 000 people, raising concerns about our preparedness against this emerging viral pathogen. Licensed and approved for mpox, the JYNNEOS vaccine has fewer side-effects than previous smallpox vaccines and has shown immunogenicity against monkeypox in animal models. This study aims to elucidate human immune responses to JYNNEOS vaccination compared with mpox-induced immunity.
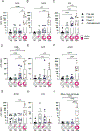
Methods: Peripheral blood mononuclear cells and sera were obtained from ten individuals vaccinated with one or two doses of JYNNEOS and six individuals diagnosed with monkeypox virus infection. Samples were obtained from seven individuals before vaccination to serve as a baseline. We examined the polyclonal serum (ELISA) and single B-cell (heavy chain gene and transcriptome data) antibody repertoires and T-cell responses (activation-induced marker and intracellular cytokine staining assays) induced by the JYNNEOS vaccine versus monkeypox virus infection.
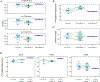
Findings: All participants were men between the ages of 21 and 60 years, except for one woman in the group of mpox-convalescent individuals, and none had previous orthopoxvirus exposure. All mpox cases were mild. Vaccinee samples were collected 6-33 days after the first dose and 5-40 days after the second dose. Mpox-convalescent samples were collected 20-102 days after infection. In vaccine recipients, gene-level plasmablast and antibody responses were negligible and sera displayed moderate binding to recombinant orthopoxviral proteins (A29L, A35R, E8L, A30L, A27L, A33R, B18R, and L1R) and native proteins from the 2022 monkeypox outbreak strain. By contrast, recent monkeypox virus infection (within 20-102 days) induced robust serum antibody responses to monkeypox virus proteins and to native monkeypox virus proteins from a viral isolate obtained during the 2022 outbreak. JYNNEOS vaccine recipients presented robust orthopoxviral CD4+ and CD8+ T-cell responses.
Interpretation: Infection with monkeypox virus resulted in robust B-cell and T-cell responses, whereas immunisation with JYNNEOS elicited more robust T-cell responses. These data can help to inform vaccine design and policies for preventing mpox in humans.
Funding: National Cancer Institute (National Institutes of Health), National Institute of Allergy and Infectious Diseases (National Institutes of Health), and Icahn School of Medicine.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests FK has consulted for Curevac, Seqirus, and Merck; is currently consulting for Pfizer, Third Rock Ventures, Avimex, and GSK; and is named on several patents regarding influenza virus and SARS-CoV-2 vaccines, influenza virus therapeutics, and SARS-CoV-2 serological tests, some of which have been licensed to commercial entities, from which FK is receiving royalties. FK is also an advisory board member of Castlevax, a spin-off company formed by the Icahn School of Medicine at Mount Sinai to develop SARS-CoV-2 vaccines. FK's laboratory has received funding for research projects from Pfizer, GSK, and Dynavax, and three of FK's mentees have recently joined Moderna. All other authors declare no competing interests.
Figures
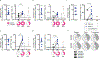
Update of
-
Mpox vaccine and infection-driven human immune signatures.medRxiv [Preprint]. 2023 Mar 9:2023.03.07.23286701. doi: 10.1101/2023.03.07.23286701. medRxiv. 2023. Update in: Lancet Infect Dis. 2023 Nov;23(11):1302-1312. doi: 10.1016/S1473-3099(23)00352-3. PMID: 36945651 Free PMC article. Updated. Preprint.
Comment in
-
Progress in the evaluation of modified vaccinia Ankara vaccine against mpox.Lancet Infect Dis. 2023 Nov;23(11):1214-1215. doi: 10.1016/S1473-3099(23)00369-9. Epub 2023 Jul 17. Lancet Infect Dis. 2023. PMID: 37475114 No abstract available.
References
-
- Zucker J CROI 2023: EPIDEMIOLOGY, DIAGNOSIS, AND MANAGEMENT OF MPOX. In 2023. [cited 2023 May 16]. p. 1–16. Available from: https://www.iasusa.org/wp-content/uploads/2023/04/30-3-zucker-v1.pdf - PMC - PubMed
-
- Bertran M, Andrews N, Davison C, Dugbazah B, Boateng J, Lunt R, et al. Effectiveness of one dose of MVA–BN smallpox vaccine against mpox in England using the case-coverage method: an observational study. Lancet Infect Dis [Internet]. 2023. Mar 13 [cited 2023 Apr 20];0(0). Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00057... - PubMed
-
- Preliminary JYNNEOS Vaccine Effectiveness Estimates Against Medically Attended Mpox Disease in the U.S., August 15, 2022 – October 29, 2022 | Mpox| Poxvirus | CDC; [Internet]. 2023. [cited 2023 Apr 20]. Available from: https://www.cdc.gov/poxvirus/mpox/cases-data/JYNNEOS-vaccine-effectivene...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

