Extent of piriform cortex resection in children with temporal lobe epilepsy
- PMID: 37475156
- PMCID: PMC10502684
- DOI: 10.1002/acn3.51852
Extent of piriform cortex resection in children with temporal lobe epilepsy
Abstract
Objective: A greater extent of resection of the temporal portion of the piriform cortex (PC) has been shown to be associated with higher likelihood of seizure freedom in adults undergoing anterior temporal lobe resection (ATLR) for drug-resistant temporal lobe epilepsy (TLE). There have been no such studies in children, therefore this study aimed to investigate this association in a pediatric cohort.
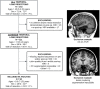
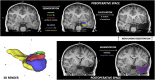
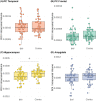
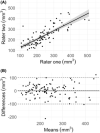
Methods: A retrospective, neuroimaging cohort study of children with TLE who underwent ATLR between 2012 and 2021 was undertaken. The PC, hippocampal and amygdala volumes were measured on the preoperative and postoperative T1-weighted MRI. Using these volumes, the extent of resection per region was compared between the seizure-free and not seizure-free groups.
Results: In 50 children (median age 9.5 years) there was no significant difference between the extent of resection of the temporal PC in the seizure-free (median = 50%, n = 33/50) versus not seizure-free (median = 40%, n = 17/50) groups (p = 0.26). In a sub-group of 19 with ipsilateral hippocampal atrophy (quantitatively defined by ipsilateral-to-contralateral asymmetry), the median extent of temporal PC resection was greater in children who were seizure-free (53%) versus those not seizure-free (19%) (p = 0.009).
Interpretation: This is the first study demonstrating that, in children with TLE and hippocampal atrophy, more extensive temporal PC resection is associated with a greater chance of seizure freedom-compatible with an adult series in which 85% of patients had hippocampal sclerosis. In a combined group of children with and without hippocampal atrophy, the extent of PC resection was not associated with seizure outcome, suggesting different epileptogenic networks within this cohort.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Association of Piriform Cortex Resection With Surgical Outcomes in Patients With Temporal Lobe Epilepsy.JAMA Neurol. 2019 Jun 1;76(6):690-700. doi: 10.1001/jamaneurol.2019.0204. JAMA Neurol. 2019. PMID: 30855662 Free PMC article.
-
Optimal Surgical Extent for Memory and Seizure Outcome in Temporal Lobe Epilepsy.Ann Neurol. 2022 Jan;91(1):131-144. doi: 10.1002/ana.26266. Epub 2021 Nov 20. Ann Neurol. 2022. PMID: 34741484 Free PMC article.
-
Volumetric analysis of the piriform cortex in temporal lobe epilepsy.Epilepsy Res. 2022 Sep;185:106971. doi: 10.1016/j.eplepsyres.2022.106971. Epub 2022 Jun 24. Epilepsy Res. 2022. PMID: 35810570 Free PMC article.
-
Apparent diffusion coefficient of piriform cortex and seizure outcome in mesial temporal lobe epilepsy after MR-guided laser interstitial thermal therapy: a single-institution experience.J Neurosurg. 2022 Apr 29;137(6):1601-1609. doi: 10.3171/2022.3.JNS212490. Print 2022 Dec 1. J Neurosurg. 2022. PMID: 35535837
-
The Little-Known Ribbon-Shaped Piriform Cortex: A Key Node in Temporal Lobe Epilepsy-Anatomical Insights and Its Potential for Surgical Treatment.Diagnostics (Basel). 2024 Dec 17;14(24):2838. doi: 10.3390/diagnostics14242838. Diagnostics (Basel). 2024. PMID: 39767200 Free PMC article. Review.
Cited by
-
Piriform cortex is an ictogenic trigger zone in the primate brain.Epilepsia. 2025 Feb;66(2):569-582. doi: 10.1111/epi.18201. Epub 2024 Dec 5. Epilepsia. 2025. PMID: 39636294
-
Relationship of left piriform cortex network centrality with temporal lobe epilepsy duration and drug resistance.Eur J Neurol. 2025 Feb;32(2):e70018. doi: 10.1111/ene.70018. Eur J Neurol. 2025. PMID: 39949073 Free PMC article.
-
Connectivity of the Piriform Cortex and its Implications in Temporal Lobe Epilepsy.medRxiv [Preprint]. 2024 Jul 22:2024.07.21.24310778. doi: 10.1101/2024.07.21.24310778. medRxiv. 2024. PMID: 39108505 Free PMC article. Preprint.
References
-
- Cross JH, Reilly C, Gutierrez Delicado E, et al. Epilepsy surgery for children and adolescents: evidence‐based but underused. Lancet Child Adolesc Heal. 2022;6(7):484‐494. - PubMed
-
- Barba C, Giometto S, Lucenteforte E, et al. Seizure outcome of temporal lobe epilepsy surgery in adults and children: a systematic review and meta‐analysis. Neurosurgery. 2022;91(5):676‐683. - PubMed
-
- Najm I, Jehi L, Palmini A, et al. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54(5):772‐782. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
