Photobiomodulation reduces neuropathic pain after spinal cord injury by downregulating CXCL10 expression
- PMID: 37475184
- PMCID: PMC10651991
- DOI: 10.1111/cns.14325
Photobiomodulation reduces neuropathic pain after spinal cord injury by downregulating CXCL10 expression
Abstract
Background: Many studies have recently highlighted the role of photobiomodulation (PBM) in neuropathic pain (NP) relief after spinal cord injury (SCI), suggesting that it may be an effective way to relieve NP after SCI. However, the underlying mechanisms remain unclear. This study aimed to determine the potential mechanisms of PBM in NP relief after SCI.
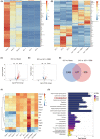
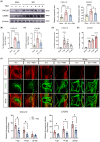
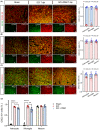
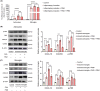
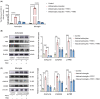
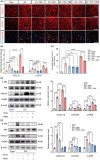
Methods: We performed systematic observations and investigated the mechanism of PBM intervention in NP in rats after SCI. Using transcriptome sequencing, we screened CXCL10 as a possible target molecule for PBM intervention and validated the results in rat tissues using reverse transcription-polymerase chain reaction and western blotting. Using immunofluorescence co-labeling, astrocytes and microglia were identified as the cells responsible for CXCL10 expression. The involvement of the NF-κB pathway in CXCL10 expression was verified using inhibitor pyrrolidine dithiocarbamate (PDTC) and agonist phorbol-12-myristate-13-acetate (PMA), which were further validated by an in vivo injection experiment.
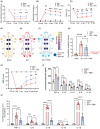
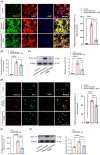
Results: Here, we demonstrated that PBM therapy led to an improvement in NP relative behaviors post-SCI, inhibited the activation of microglia and astrocytes, and decreased the expression level of CXCL10 in glial cells, which was accompanied by mediation of the NF-κB signaling pathway. Photobiomodulation inhibit the activation of the NF-κB pathway and reduce downstream CXCL10 expression. The NF-κB pathway inhibitor PDTC had the same effect as PBM on improving pain in animals with SCI, and the NF-κB pathway promoter PMA could reverse the beneficial effect of PBM.
Conclusions: Our results provide new insights into the mechanisms by which PBM alleviates NP after SCI. We demonstrated that PBM significantly inhibited the activation of microglia and astrocytes and decreased the expression level of CXCL10. These effects appear to be related to the NF-κB signaling pathway. Taken together, our study provides evidence that PBM could be a potentially effective therapy for NP after SCI, CXCL10 and NF-kB signaling pathways might be critical factors in pain relief mediated by PBM after SCI.
Keywords: CXCL10; glia cells; neuropathic pain; photobiomodulation; spinal cord injury.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Neuropathic Pain Induced by Spinal Cord Injury from the Glia Perspective and Its Treatment.Cell Mol Neurobiol. 2024 Nov 28;44(1):81. doi: 10.1007/s10571-024-01517-x. Cell Mol Neurobiol. 2024. PMID: 39607514 Free PMC article. Review.
-
Systematic analysis of critical genes and pathways identified a signature of neuropathic pain after spinal cord injury.Eur J Neurosci. 2022 Jul;56(2):3991-4008. doi: 10.1111/ejn.15693. Epub 2022 May 27. Eur J Neurosci. 2022. PMID: 35560852 Review.
-
Photobiomodulation inhibits the activation of neurotoxic microglia and astrocytes by inhibiting Lcn2/JAK2-STAT3 crosstalk after spinal cord injury in male rats.J Neuroinflammation. 2021 Nov 5;18(1):256. doi: 10.1186/s12974-021-02312-x. J Neuroinflammation. 2021. PMID: 34740378 Free PMC article.
-
Synergistic effects of tetramethylpyrazine and astragaloside IV on spinal cord injury via alteration of astrocyte A1/A2 polarization through the Sirt1-NF-κB pathway.Int Immunopharmacol. 2024 Apr 20;131:111686. doi: 10.1016/j.intimp.2024.111686. Epub 2024 Mar 10. Int Immunopharmacol. 2024. PMID: 38461631
-
Photobiomodulation Attenuates Neurotoxic Polarization of Macrophages by Inhibiting the Notch1-HIF-1α/NF-κB Signalling Pathway in Mice With Spinal Cord Injury.Front Immunol. 2022 Mar 17;13:816952. doi: 10.3389/fimmu.2022.816952. eCollection 2022. Front Immunol. 2022. PMID: 35371065 Free PMC article.
Cited by
-
Neuropathic Pain Induced by Spinal Cord Injury from the Glia Perspective and Its Treatment.Cell Mol Neurobiol. 2024 Nov 28;44(1):81. doi: 10.1007/s10571-024-01517-x. Cell Mol Neurobiol. 2024. PMID: 39607514 Free PMC article. Review.
-
NEMO-Binding Domain/IKKγ Inhibitory Peptide Alleviates Neuronal Pyroptosis in Spinal Cord Injury by Inhibiting ASMase-Induced Lysosome Membrane Permeabilization.Adv Sci (Weinh). 2024 Oct;11(40):e2405759. doi: 10.1002/advs.202405759. Epub 2024 Sep 3. Adv Sci (Weinh). 2024. PMID: 39225315 Free PMC article.
-
Role of microglia in diabetic neuropathic pain.Front Cell Dev Biol. 2024 Jul 29;12:1421191. doi: 10.3389/fcell.2024.1421191. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39135776 Free PMC article. Review.
-
Autophagy modulation by hADSCs and green light therapy alleviates inflammation and promotes functional recovery after spinal cord injury.Stem Cell Res Ther. 2025 May 22;16(1):256. doi: 10.1186/s13287-025-04367-6. Stem Cell Res Ther. 2025. PMID: 40405285 Free PMC article.
-
Tomatidine relieves neuronal damage in spinal cord injury by inhibiting the inflammatory responses and apoptosis through blocking the NF-κB/CXCL10 pathway activation.Front Pharmacol. 2024 Dec 12;15:1503925. doi: 10.3389/fphar.2024.1503925. eCollection 2024. Front Pharmacol. 2024. PMID: 39726790 Free PMC article.
References
-
- Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: a systematic review and meta‐analysis. Eur J Pain. 2017;21(1):29‐44. - PubMed
-
- Finnerup N, Norrbrink C, Trok K, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain. 2014;15(1):40‐48. - PubMed
-
- Burke D, Lennon O, Fullen BM. Quality of life after spinal cord injury: the impact of pain. Eur J Pain. 2018;22(9):1662‐1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

