What determines the optimal pharmacological treatment of atrial fibrillation? Insights from in silico trials in 800 virtual atria
- PMID: 37475475
- PMCID: PMC10952228
- DOI: 10.1113/JP284730
What determines the optimal pharmacological treatment of atrial fibrillation? Insights from in silico trials in 800 virtual atria
Abstract
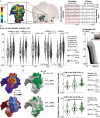
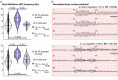
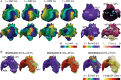
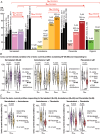
The best pharmacological treatment for each atrial fibrillation (AF) patient is unclear. We aim to exploit AF simulations in 800 virtual atria to identify key patient characteristics that guide the optimal selection of anti-arrhythmic drugs. The virtual cohort considered variability in electrophysiology and low voltage areas (LVA) and was developed and validated against experimental and clinical data from ionic currents to ECG. AF sustained in 494 (62%) atria, with large inward rectifier K+ current (IK1 ) and Na+ /K+ pump (INaK ) densities (IK1 0.11 ± 0.03 vs. 0.07 ± 0.03 S mF-1 ; INaK 0.68 ± 0.15 vs. 0.38 ± 26 S mF-1 ; sustained vs. un-sustained AF). In severely remodelled left atrium, with LVA extensions of more than 40% in the posterior wall, higher IK1 (median density 0.12 ± 0.02 S mF-1 ) was required for AF maintenance, and rotors localized in healthy right atrium. For lower LVA extensions, rotors could also anchor to LVA, in atria presenting short refractoriness (median L-type Ca2+ current, ICaL , density 0.08 ± 0.03 S mF-1 ). This atrial refractoriness, modulated by ICaL and fast Na+ current (INa ), determined pharmacological treatment success for both small and large LVA. Vernakalant was effective in atria presenting long refractoriness (median ICaL density 0.13 ± 0.05 S mF-1 ). For short refractoriness, atria with high INa (median density 8.92 ± 2.59 S mF-1 ) responded more favourably to amiodarone than flecainide, and the opposite was found in atria with low INa (median density 5.33 ± 1.41 S mF-1 ). In silico drug trials in 800 human atria identify inward currents as critical for optimal stratification of AF patient to pharmacological treatment and, together with the left atrial LVA extension, for accurately phenotyping AF dynamics. KEY POINTS: Atrial fibrillation (AF) maintenance is facilitated by small L-type Ca2+ current (ICaL ) and large inward rectifier K+ current (IK1 ) and Na+ /K+ pump. In severely remodelled left atrium, with low voltage areas (LVA) covering more than 40% of the posterior wall, sustained AF requires higher IK1 and rotors localize in healthy right atrium. For lower LVA extensions, rotors can also anchor to LVA, if the atria present short refractoriness (low ICaL ) Vernakalant is effective in atria presenting long refractoriness (high ICaL ). For short refractoriness, atria with fast Na+ current (INa ) up-regulation respond more favourably to amiodarone than flecainide, and the opposite is found in atria with low INa . The inward currents (ICaL and INa ) are critical for optimal stratification of AF patient to pharmacological treatment and, together with the left atrial LVA extension, for accurately phenotyping AF dynamics.
Keywords: atrial fibrillation; in silico drug trials; ionic currents; low voltage areas.
© 2023 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Comment in
-
Overcoming a combinatorial explosion: What we can learn from 5928 atrial fibrillation anti-arrhythmic drug simulations.J Physiol. 2023 Sep;601(18):3985-3986. doi: 10.1113/JP285252. Epub 2023 Aug 26. J Physiol. 2023. PMID: 37632721 No abstract available.
References
-
- Alcaraz, R. , Sandberg, F. , Sörnmo, L. , & Rieta, J. J. (2011). Classification of paroxysmal and persistent atrial fibrillation in ambulatory ECG recordings. IEEE Transactions on Biomedical Engineering, 58(5), 1441–1449. - PubMed
-
- Atienza, F. , Almendral, J. , Moreno, J. , Vaidyanathan, R. , Talkachou, A. , Kalifa, J. , Arenal, A. , Villacastín, J. P. , Torrecilla, E. G. , Sánchez, A. , Ploutz‐Snyder, R. , Jalife, J. , & Berenfeld, O. (2006). Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation, 114(23), 2434–2442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

