Peptide Macrocyclization Guided by Reversible Covalent Templating
- PMID: 37475574
- PMCID: PMC10592230
- DOI: 10.1002/chem.202301949
Peptide Macrocyclization Guided by Reversible Covalent Templating
Abstract
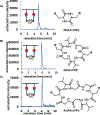
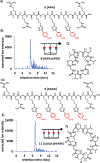
The creation of complementary products via templating is a hallmark feature of nucleic acid replication. Outside of nucleic acid-like molecules, the templated synthesis of a hetero-complementary copy is still rare. Herein we describe one cycle of templated synthesis that creates homomeric macrocyclic peptides guided by linear instructing strands. This strategy utilizes hydrazone formation to pre-organize peptide oligomeric monomers along the template on a solid support resin, and microwave-assisted peptide synthesis to couple monomers and cyclize the strands. With a flexible templating strand, we can alter the size of the complementary macrocycle products by increasing the length and number of the binding peptide oligomers, showing the potential to precisely tune the size of macrocyclic products. For the smaller macrocyclic peptides, the products can be released via hydrolysis and characterized by ESI-MS.
Keywords: dynamic covalent bonds; peptides; replication; sequence defined; templating.
© 2023 Wiley-VCH GmbH.
Figures

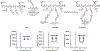

References
-
- Campbell MK, Farrell SO, Mcdougal OM Biochemistry, 9th ed., Cengage Learning: Boston, Ma, 2018, pp 245.
-
- Leguizamon SC, Scott TF, Pol. Rev. 2022, 62, 626–651;
- Leguizamon SC, Alqubati AF Scott TF, Polym. Chem. 2020, 11, 7714–7720;
- Meyer AJ, Ellefson JW, Ellington AD, Acc. Chem. Res. 2012, 45, 2097–2105. - PubMed
-
- Hill DJ, Mio MJ, Prince RB, Hughes TS, Moore JS, Chem. Rev. 2001, 101, 3893–4012; - PubMed
- Gong B, Acc. Chem. Res. 2000, 122, 8856–8868;
- Li Z-T, Wu L-Z, Springer: Berlin 2015, 115–136;
- Troselj P, Bolgar P, Ballester P, Hunter CA, J. Am. Chem. Soc 2021, 143, 8669–8678; - PMC - PubMed
- Núñez-Villanueva D, Hunter CA, Acc. Chem. Res. 2021, 54, 1298–1306. - PMC - PubMed
-
- Koert U, Harding MM, Lehn J-M, Nature 1990, 346, 339–342; - PubMed
- Chmielewski MJ, Buhler E, Candau J, Lehn J-M, Chem. Eur. Jour. 2014, 20, 6960–6977; - PubMed
- Zhao D, Van Leeuwen T, Cheng J, Feringa BL, Nat. Chem. 2017, 9, 250–256; - PubMed
- Ashbridge Z, Kreidt E, Pirvu L, Schaufelberger F, Stenlid JH, Abild-Pedersen F, Leigh DA Science 2022, 375 (6584), 1035–1041; - PubMed
- Fasano F, Bolgar P, Iadevaia G, Hunter CA, Chem. Sci. 2022, 13 (44), 13085. - PMC - PubMed
- Núñez-Villanueva D, Hunter CA, Org. Biomol. Chem, 2022,20, 8285–8292. - PMC - PubMed
- Núñez-Villanueva D, Hunter CA, J. Am. Chem. Soc. 2022, 144, 17307−17316. - PMC - PubMed
-
- Dunn MF, Wei T, Zuckerman RN, Scott TF Polym. Chem. 2019, 10, 2337–2343.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

