Pregnancy related hormones increase CYP3A mediated buprenorphine metabolism in human hepatocytes: a comparison to CYP3A substrates nifedipine and midazolam
- PMID: 37475714
- PMCID: PMC10354249
- DOI: 10.3389/fphar.2023.1218703
Pregnancy related hormones increase CYP3A mediated buprenorphine metabolism in human hepatocytes: a comparison to CYP3A substrates nifedipine and midazolam
Abstract
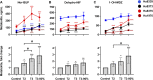
Introduction: Pregnancy increases the clearance of CYP3A4 substrate drugs and pregnancy-related hormones (PRHs) induce hepatic CYP3A4 expression and metabolism. However, it remains unclear to what extent the magnitude of PRH-evoked changes in hepatic CYP3A metabolism varies across multiple substrates. This study quantified the impact of PRHs on CYP3A protein concentrations and buprenorphine metabolism in human hepatocytes, and compared the magnitude of these effects to nifedipine and midazolam metabolism. Methods: Sandwich-cultured human hepatocytes (SCHH) from female donors were exposed to PRHs, administered in combination across a range of physiologically relevant concentrations, for 72 h. Absolute protein concentrations of CYP3A4, CYP3A5, and CYP3A7 in SCHH membrane fractions were quantified by nanoLC-MS/MS, and norbuprenorphine (nor-BUP), dehydro-nifedipine (dehydro-NIF), and 1-hydroxy-midazolam (1-OH-MDZ) formation was evaluated. Results: Compared to control, PRH exposure increased CYP3A4, CYP3A7, and total CYP3A protein concentrations, but not CYP3A5 concentrations, and increased nor-BUP, dehydro-NIF, and 1-OH-MDZ formation in a concentration-dependent manner. The formation of nor-BUP, dehydro-NIF, and 1-OH-MDZ each positively correlated with PRH-mediated changes in total CYP3A protein concentrations. The PRH-evoked increase in nor-BUP formation was evident in all donors; however, the PRH induction of dehydro-NIF and 1-OH-MDZ formation was diminished in a hepatocyte donor with high basal CYP3A5 expression. Discussion: These findings demonstrate that PRHs increase buprenorphine, nifedipine, and midazolam metabolism in SCHH via induction of CYP3A4 and total CYP3A protein concentrations, and the magnitude of these effects vary across hepatocyte donors in a substrate-specific manner. These data provide insight into the contribution of PRH induction of CYP3A4 metabolism to increased buprenorphine clearance during pregnancy.
Keywords: CYP3A4; CYP3A5; buprenorphine; hepatic metabolism; nifedipine; pregnancy; pregnancy related hormones; targeted proteomics.
Copyright © 2023 Fashe, Miner, Fallon, Schauer, Sykes, Smith and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Pregnancy-Related Hormones Increase Nifedipine Metabolism in Human Hepatocytes by Inducing CYP3A4 Expression.J Pharm Sci. 2021 Jan;110(1):412-421. doi: 10.1016/j.xphs.2020.09.013. Epub 2020 Sep 12. J Pharm Sci. 2021. PMID: 32931777 Free PMC article.
-
Impact of sex and pregnancy on hepatic CYP3A4 expression and activity in a humanized mouse model.Drug Metab Dispos. 2025 Feb;53(2):100025. doi: 10.1016/j.dmd.2024.100025. Epub 2024 Nov 29. Drug Metab Dispos. 2025. PMID: 40023573
-
Association of NR1I2 Polymorphism with Midazolam Clearance in Mechanically Ventilated ICU Patients: A Population Pharmacokinetic and Pharmacogenetic Study.Drug Des Devel Ther. 2025 Mar 4;19:1527-1541. doi: 10.2147/DDDT.S495647. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40066084 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Effectiveness and safety of vitamin D in relation to bone health.Evid Rep Technol Assess (Full Rep). 2007 Aug;(158):1-235. Evid Rep Technol Assess (Full Rep). 2007. PMID: 18088161 Free PMC article.
Cited by
-
Mechanisms of altered hepatic drug disposition during pregnancy: small molecules.Expert Opin Drug Metab Toxicol. 2025 Apr;21(4):445-462. doi: 10.1080/17425255.2025.2470792. Epub 2025 Feb 26. Expert Opin Drug Metab Toxicol. 2025. PMID: 39992297 Review.
-
Inclusivity in Epilepsy Care: Navigating the Complex Nature of Seizure Disorders in People Undergoing Gender-Affirming Care.Epilepsy Curr. 2025 Mar 2;25(4):253-262. doi: 10.1177/15357597251317908. eCollection 2025 Jul-Aug. Epilepsy Curr. 2025. PMID: 40040857 Free PMC article. Review.
-
Impact of Pregnancy on the Pharmacokinetics and Metabolism of Nicotinamide in Humans.Clin Pharmacol Ther. 2024 Mar;115(3):556-564. doi: 10.1002/cpt.3146. Epub 2024 Jan 11. Clin Pharmacol Ther. 2024. PMID: 38093631 Free PMC article.
-
Population pharmacokinetics of amodiaquine and piperaquine in African pregnant women with uncomplicated Plasmodium falciparum infections.CPT Pharmacometrics Syst Pharmacol. 2024 Nov;13(11):1893-1903. doi: 10.1002/psp4.13211. Epub 2024 Sep 3. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 39228131 Free PMC article. Clinical Trial.
-
Physiologically Based Pharmacokinetic Modeling in Pregnancy, during Lactation and in Neonates: Achievements, Challenges and Future Directions.Pharmaceutics. 2024 Apr 5;16(4):500. doi: 10.3390/pharmaceutics16040500. Pharmaceutics. 2024. PMID: 38675161 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

