Infiltrated IL-17A-producing gamma delta T cells play a protective role in sepsis-induced liver injury and are regulated by CCR6 and gut commensal microbes
- PMID: 37475963
- PMCID: PMC10354519
- DOI: 10.3389/fcimb.2023.1149506
Infiltrated IL-17A-producing gamma delta T cells play a protective role in sepsis-induced liver injury and are regulated by CCR6 and gut commensal microbes
Abstract
Introduction: Sepsis is a common but serious disease in intensive care units, which may induce multiple organ dysfunctions such as liver injury. Previous studies have demonstrated that gamma delta (γδ) T cells play a protective role in sepsis. However, the function and mechanism of γδ T cells in sepsis-induced liver injury have not been fully elucidated. IL-17A-producing γδ T cells are a newly identified cell subtype.
Methods: We utilized IL-17A-deficient mice to investigate the role of IL-17A-producing γδ T cells in sepsis using the cecum ligation and puncture (CLP) model.
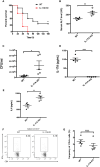
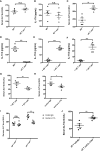
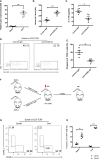
Results: Our findings suggested that these cells were the major source of IL-17A and protected against sepsis-induced liver injury. Flow cytometry analysis revealed that these γδ T cells expressed Vγ4 TCR and migrated into liver from peripheral post CLP, in a CCR6-dependent manner. When CLP mice were treated with anti-CCR6 antibody to block CCR6-CCL20 axis, the recruitment of Vγ4+ γδ T cells was abolished, indicating a CCR6-dependent manner of migration. Interestingly, pseudo germ-free CLP mice treated with antibiotics showed that hepatic IL-17A+ γδ T cells were regulated by gut commensal microbes. E. coli alone were able to restore the protective effect in pseudo germ-free mice by rescuing hepatic IL-17A+ γδ T cell population.
Conclusion: Our research has shown that Vγ4+ IL-17A+ γδ T cells infiltrating into the liver play a crucial role in protecting against sepsis-induced liver injury. This protection was contingent upon the recruitment of CCR6 and regulated by gut commensal microbes.
Keywords: CCR6; IL-17A; gamma delta T cells; liver injury; microbiota; sepsis.
Copyright © 2023 Wan, Zhang, Hao, Tao, Song, Chen, Qin, Song and Shan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

