The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- PMID: 37475970
- PMCID: PMC10324924
- DOI: 10.4078/jrd.2022.29.3.140
The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
Abstract
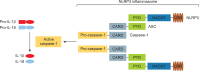
The NACHT, LRR, and PYD-domains-containing protein 3 (NLRP3) inflammasome is an intracellular multi-protein signaling platform that is activated by cytosolic pattern-recognition receptors such as NLRs against endogenous and exogenous pathogens. Once it is activated by a variety of danger signals, recruitment and assembly of NLRP3, ASC, and pro-caspase-1 trigger the processing and release of pro-inflammatory cytokines including interleukin-1β (IL-1β) and IL-18. Multiple intracellular and extracellular structures and molecular mechanisms are involved in NLRP3 inflammasome activation. Gout is an autoinflammatory disease induced by inflammatory response through production of NLRP3 inflammasome-mediated proinflammatory cytokines such as IL-1β by deposition of monosodium urate (MSU) crystals in the articular joints and periarticular structures. NLRP3 inflammasome is considered a main therapeutic target in MSU crystal-induced inflammation in gout. Novel therapeutic strategies have been proposed to control acute flares of gouty arthritis and prophylaxis for gout flares through modulation of the NLRP3/IL-1 axis pathway. This review discusses the basic mechanism of NLRP3 inflammasome activation and the IL-1-induced inflammatory cascade and explains the NLRP3 inflammasome-induced pathogenic role in the pathogenesis of gout.
Keywords: Gout; Inflammasome; Interleukin-1; Monosodium urate; NLRP3.
Copyright © 2022 by The Korean College of Rheumatology. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Figures


References
-
- Freudweiler M. Experimentelle untersuchungen uber das wesen der gichtknoten. Dtsch Arch Klin Med. 1899;63:266–335. German.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
