Use of Disease-modifying Antirheumatic Drugs After Cancer Diagnosis in Rheumatoid Arthritis Patients
- PMID: 37475975
- PMCID: PMC10324922
- DOI: 10.4078/jrd.2022.29.3.162
Use of Disease-modifying Antirheumatic Drugs After Cancer Diagnosis in Rheumatoid Arthritis Patients
Abstract
Objective: There is no recommendation for the use of disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis (RA) who developed cancer. We examined changes in the DMARDs prescription patterns associated with cancer diagnosis in RA patients.
Methods: We reviewed the medical records of 2,161 RA patients who visited rheumatology clinic between January 2008 and February 2017 and found 40 patients who developed cancer during RA treatment. In these patients, we examined DMARDs prescription patterns before and right after cancer diagnosis and at recent outpatient clinic visits.
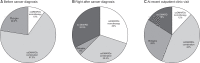
Results: Before cancer diagnosis, methotrexate (MTX)-combined conventional synthetic DMARDs (csDMARDs) were most commonly prescribed (22, 55.0%) and biological DMARDs (biologics) in nine patients (22.5%). For cancer treatment, 19 patients received chemotherapy (including adjuvant chemotherapy) and 21 patients had surgery only. Right after cancer diagnosis, changes in the DMARDs prescription patterns were similar in discontinuation (13, 32.5%), switching (14, 35.0%), and maintenance (13, 32.5%). DMARDs were discontinued more frequently in the chemotherapy group (9/19, 47.4%) than the surgery only group (4/2, 19.0%) (p<0.05). Among the 13 patients who discontinued DMARDs, nine (69.2%) resumed DMARDs after a median of 5.5 months (interquartile range [IQR] 2.9, 18.3) due to arthritis flare. At a median of 4.6 years (IQR 3.3, 6.7) after cancer diagnosis, 25 patients were evaluated at recent outpatient clinic visits. Four patients received no DMARD, three MTX monotherapies, 11 csDMARDs combination therapies, and seven biologics.
Conclusion: A significant number of RA patients who developed cancer during RA treatment were still receiving DMARDs including biologics after cancer diagnosis.
Keywords: Biologics; Cancer; Disease-modifying antirheumatic drugs; Methotrexate; Rheumatoid arthritis.
Copyright © 2022 by The Korean College of Rheumatology. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Khurana R, Wolf R, Berney S, Caldito G, Hayat S, Berney SM. Risk of development of lung cancer is increased in patients with rheumatoid arthritis: a large case control study in US veterans. J Rheumatol. 2008;35:1704–8. - PubMed
-
- Lopez-Olivo MA, Colmegna I, Karpes Matusevich AR, Qi SR, Zamora NV, Sharma R, et al. Systematic review of recommendations on the use of disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and cancer. Arthritis Care Res (Hoboken) 2020;72:309–18. doi: 10.1002/acr.23865. - DOI - PubMed
-
- Strangfeld A, Hierse F, Rau R, Burmester GR, Krummel-Lorenz B, Demary W, et al. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther. 2010;12:R5. doi: 10.1186/ar2904. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
