Locus coeruleus inhibition of vibrissal responses in the trigeminal subnucleus caudalis are reduced in a diabetic mouse model
- PMID: 37475984
- PMCID: PMC10354250
- DOI: 10.3389/fncel.2023.1208121
Locus coeruleus inhibition of vibrissal responses in the trigeminal subnucleus caudalis are reduced in a diabetic mouse model
Abstract
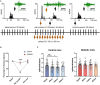
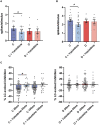
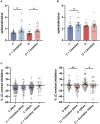
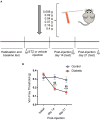
Diabetic neuropathy is the loss of sensory function beginning distally in the lower extremities, which is also characterized by pain and substantial morbidity. Furthermore, the locus coeruleus (LC) nucleus has been proposed to play an important role in descending pain control through the activation of α2-noradrenergic (NA) receptors in the spinal dorsal horn. We studied, on control and diabetic mice, the effect of electrical stimulation of the LC nucleus on the tactile responses in the caudalis division of the spinal trigeminal nucleus (Sp5C), which is involved in the relay of orofacial nociceptive information. Diabetes was induced in young adult C57BL/6J mice with one intraperitoneal injection of streptozotocin (50 mg/kg) daily for 5 days. The diabetic animals showed pain in the orofacial area because they had a decrease in the withdrawal threshold to the mechanical stimulation in the vibrissal pad. LC electrical stimulation induced the inhibition of vibrissal responses in the Sp5C neurons when applied at 50 and 100 ms before vibrissal stimulation in the control mice; however, the inhibition was reduced in the diabetic mice. These effects may be due to a reduction in the tyrosine hydroxylase positive (TH+) fibers in the Sp5C, as was observed in diabetic mice. LC-evoked inhibition was decreased by an intraperitoneal injection of the antagonist of the α2-NA receptors, yohimbine, indicating that it was due to the activation of α2-NA receptors. The decrease in the LC-evoked inhibition in the diabetic mice was partially recovered when clonidine, a non-selective α2-agonist, was injected intraperitoneally. These findings suggest that in diabetes, there is a reduction in the NA inputs from the LC in the Sp5C that may favor the development of chronic pain.
Keywords: clonidine; diabetes; hyperglycemia; neuropathic pain; noradrenergic transmission; yohimbine; α2-noradrenergic receptor.
Copyright © 2023 Mesa-Lombardo, García-Magro, Nuñez and Martin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bae Y. C., Ihn H. J., Park M. J., Ottersen O. P., Moritani M., Yoshida A., et al. (2000). Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J. Comp. Neurol. 418, 299–309. 10.1002/(SICI)1096-9861(20000313)418:3andlt;299::AID-CNE5andgt;3.0.CO;2-I - DOI - PubMed
LinkOut - more resources
Full Text Sources

