Digital therapeutics in the clinic
- PMID: 37476062
- PMCID: PMC10354777
- DOI: 10.1002/btm2.10536
Digital therapeutics in the clinic
Abstract
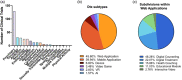
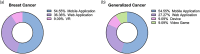
Digital therapeutics are emerging as a new form of therapeutic interventions. Unlike conventional therapeutics, digital therapeutics deliver interventions directly to patients using an evidence-based, clinically evaluated software to treat, manage, or prevent diseases. Digital therapeutics manifest in diverse forms such as web-based applications, mobile applications on smart devices, virtual reality, and video games. As its own product category for FDA approval, digital therapeutics can function as stand-alone treatments or in combination with conventional therapeutics to improve adherence and/or efficacy. Here, we review the clinical landscape of digital therapeutics. We summarize FDA-approved products and their clinical use, overview >300 ongoing clinical trials, and discuss challenges for their clinical translation and strategies to overcome the same.
Keywords: digiceuticals; digital counseling; digital health; digital medicine; digital technology; digital therapeutics; prescription digital therapeutic.
© 2023 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Understanding DTx. Accessed January 13, 2023. https://dtxalliance.org/understanding-dtx/
-
- Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L. Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetes Care. 2019;43(1):250‐260. - PubMed
-
- FDA . FDA launches the digital health center of excellence. 2020. FDA.gov.
-
- FDA . Digital Health Software Precertification (Pre‐Cert) Pilot Program. 2022. Accessed September 13, 2022 https://www.fda.gov/medical-devices/digital-health-center-excellence/dig...
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

