Vat photopolymerization 3D printed microfluidic devices for organ-on-a-chip applications
- PMID: 37476860
- PMCID: PMC10448871
- DOI: 10.1039/d3lc00094j
Vat photopolymerization 3D printed microfluidic devices for organ-on-a-chip applications
Abstract
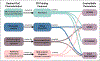
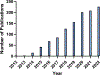
Organs-on-a-chip, or OoCs, are microfluidic tissue culture devices with micro-scaled architectures that repeatedly achieve biomimicry of biological phenomena. They are well positioned to become the primary pre-clinical testing modality as they possess high translational value. Current methods of fabrication have facilitated the development of many custom OoCs that have generated promising results. However, the reliance on microfabrication and soft lithographic fabrication techniques has limited their prototyping turnover rate and scalability. Additive manufacturing, known commonly as 3D printing, shows promise to expedite this prototyping process, while also making fabrication easier and more reproducible. We briefly introduce common 3D printing modalities before identifying two sub-types of vat photopolymerization - stereolithography (SLA) and digital light processing (DLP) - as the most advantageous fabrication methods for the future of OoC development. We then outline the motivations for shifting to 3D printing, the requirements for 3D printed OoCs to be competitive with the current state of the art, and several considerations for achieving successful 3D printed OoC devices touching on design and fabrication techniques, including a survey of commercial and custom 3D printers and resins. In all, we aim to form a guide for the end-user to facilitate the in-house generation of 3D printed OoCs, along with the future translation of these important devices.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest relating to this publication. One of the authors (G.P.N.) owns shares in Acrea 3D, a company commercializing microfluidic 3D printing.
Figures


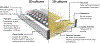

References
-
- Picollet-D’hahan N, Zuchowska A, Lemeunier I and Gac SL, Trends in Biotechnology, 2021, 39, 788–810. - PubMed
-
- Strelez C, Jiang HY and Mumenthaler SM, Trends in Biotechnology, 2023, 41, 278–280. - PubMed
-
- Berthier E, Young EWK and Beebe D, Lab on a Chip, 2012, 12, 1224. - PubMed
-
- Prabhakar P, Sen RK, Dwivedi N, Khan R, Solanki PR, Srivastava AK and Dhand C, Frontiers in Nanotechnology, 2021, 3, 1–16.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

