Adverse Effect of Neurogenic, Infective, and Inflammatory Fever on Acutely Injured Human Spinal Cord
- PMID: 37476968
- PMCID: PMC11265769
- DOI: 10.1089/neu.2023.0026
Adverse Effect of Neurogenic, Infective, and Inflammatory Fever on Acutely Injured Human Spinal Cord
Abstract
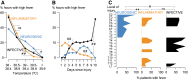
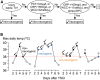
This study aims to determine the effect of neurogenic, inflammatory, and infective fevers on acutely injured human spinal cord. In 86 patients with acute, severe traumatic spinal cord injuries (TSCIs; American Spinal Injury Association Impairment Scale (AIS), grades A-C) we monitored (starting within 72 h of injury, for up to 1 week) axillary temperature as well as injury site cord pressure, microdialysis (MD), and oxygen. High fever (temperature ≥38°C) was classified as neurogenic, infective, or inflammatory. The effect of these three fever types on injury-site physiology, metabolism, and inflammation was studied by analyzing 2864 h of intraspinal pressure (ISP), 1887 h of MD, and 840 h of tissue oxygen data. High fever occurred in 76.7% of the patients. The data show that temperature was higher in neurogenic than non-neurogenic fever. Neurogenic fever only occurred with injuries rostral to vertebral level T4. Compared with normothermia, fever was associated with reduced tissue glucose (all fevers), increased tissue lactate to pyruvate ratio (all fevers), reduced tissue oxygen (neurogenic + infective fevers), and elevated levels of pro-inflammatory cytokines/chemokines (infective fever). Spinal cord metabolic derangement preceded the onset of infective but not neurogenic or inflammatory fever. By considering five clinical characteristics (level of injury, axillary temperature, leukocyte count, C-reactive protein [CRP], and serum procalcitonin [PCT]), it was possible to confidently distinguish neurogenic from non-neurogenic high fever in 59.3% of cases. We conclude that neurogenic, infective, and inflammatory fevers occur commonly after acute, severe TSCI and are detrimental to the injured spinal cord with infective fever being the most injurious. Further studies are required to determine whether treating fever improves outcome. Accurately diagnosing neurogenic fever, as described, may reduce unnecessary septic screens and overuse of antibiotics in these patients.
Keywords: antipyretic; autonomic; critical care; management; monitoring; pyrexia.
Figures
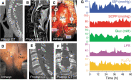

 ), no change (
), no change ( ), or decrease (
), or decrease ( ) in GLUC, tissue LPR, and psctO2. P < 0.05*, 0.005***, 0.0001###. GLUC, tissue glucose; LPR, lactate to pyruvate ratio; psctO2, tissue oxygen.
) in GLUC, tissue LPR, and psctO2. P < 0.05*, 0.005***, 0.0001###. GLUC, tissue glucose; LPR, lactate to pyruvate ratio; psctO2, tissue oxygen.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

