Production of biochar from Melia azedarach seeds for the crystal violet dye removal from water: combining of hydrothermal carbonization and pyrolysis
- PMID: 37477231
- PMCID: PMC10364649
- DOI: 10.1080/21655979.2023.2236843
Production of biochar from Melia azedarach seeds for the crystal violet dye removal from water: combining of hydrothermal carbonization and pyrolysis
Abstract
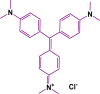
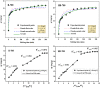
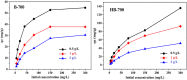
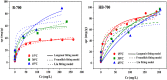
Biochar has shown large potential in water treatment because of its low cost, good textural properties, and high reusability. In this study, two porous biochars were developed from the Melia azedarach seeds via direct pyrolysis process (B-700) and through hydrothermal carbonization followed with pyrolysis (HB-700). They were characterized by morphology, structural characteristics, and surface features and used to adsorb the crystal violet (CV) dye in water environment. Results of the isotherm approaches demonstrated that the removal capacity of these biochars reached 119.4 mg/g for B-700, and 209 mg/g for HB-700 (at 45°C). Also, the Avrami model best fitted the kinetic data. The electrostatic attraction was regarded as one of the adsorptions mechanisms of CV dye. The regeneration tests reveal that both B-700 and HB-700 are good reusable adsorbents. Finally, findings of the study showed that the hydrothermal carbonization method that precede the pyrolysis process can improve significantly the adsorption capacity of the produced biochar.
Keywords: Melia azedarach seeds; biochar; carbonization; dye removal; pyrolysis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Tran HN, Wang YF, You SJ, et al. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of Π–Π interactions. Process SafEnviron Prot. 2017;107:168–180. doi: 10.1016/j.psep.2017.02.010 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
