Accelerated -Rule-Out of acute Myocardial Infarction using prehospital copeptin and in-hospital troponin: The AROMI study
- PMID: 37477353
- PMCID: PMC10568000
- DOI: 10.1093/eurheartj/ehad447
Accelerated -Rule-Out of acute Myocardial Infarction using prehospital copeptin and in-hospital troponin: The AROMI study
Abstract
Aims: The present acute myocardial infarction (AMI) rule-out strategies are challenged by the late temporal release of cardiac troponin. Copeptin is a non-specific biomarker of endogenous stress and rises early in AMI, covering the early period where troponin is still normal. An accelerated dual-marker rule-out strategy combining prehospital copeptin and in-hospital high-sensitivity troponin T could reduce length of hospital stay and thus the burden on the health care systems worldwide. The AROMI trial aimed to evaluate if the accelerated dual-marker rule-out strategy could safely reduce length of stay in patients discharged after early rule-out of AMI.
Methods and results: Patients with suspected AMI transported to hospital by ambulance were randomized 1:1 to either accelerated rule-out using copeptin measured in a prehospital blood sample and high-sensitivity troponin T measured at arrival to hospital or to standard rule-out using a 0 h/3 h rule-out strategy. The AROMI study included 4351 patients with suspected AMI. The accelerated dual-marker rule-out strategy reduced mean length of stay by 0.9 h (95% confidence interval 0.7-1.1 h) in patients discharged after rule-out of AMI and was non-inferior regarding 30-day major adverse cardiac events when compared to standard rule-out (absolute risk difference -0.4%, 95% confidence interval -2.5 to 1.7; P-value for non-inferiority = 0.013).
Conclusion: Accelerated dual marker rule-out of AMI, using a combination of prehospital copeptin and first in-hospital high-sensitivity troponin T, reduces length of hospital stay without increasing the rate of 30-day major adverse cardiac events as compared to using a 0 h/3 h rule-out strategy.
Trial registration: ClinicalTrials.gov NCT02666326.
Keywords: Acute myocardial infarction; Chest pain; Copeptin; Diagnosis; Diagnostic tests; Early diagnosis; High-sensitivity cardiac troponin; Myocardial infarction; NSTEMI; Non–ST-elevation myocardial infarction; Rule out; Rule-out; Troponin.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

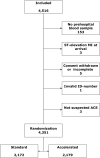
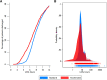

Comment in
-
Ready for rapid rule-out of acute myocardial infarction.Eur Heart J. 2023 Oct 12;44(38):3889-3891. doi: 10.1093/eurheartj/ehad519. Eur Heart J. 2023. PMID: 37592857 Free PMC article.
References
-
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. - DOI - PubMed

