Combining electromyography and Raman spectroscopy: optical EMG
- PMID: 37477391
- PMCID: PMC10952815
- DOI: 10.1002/mus.27937
Combining electromyography and Raman spectroscopy: optical EMG
Abstract
Introduction/aims: Electromyography (EMG) remains a key component of the diagnostic work-up for suspected neuromuscular disease, but it does not provide insight into the molecular composition of muscle which can provide diagnostic information. Raman spectroscopy is an emerging neuromuscular biomarker capable of generating highly specific, molecular fingerprints of tissue. Here, we present "optical EMG," a combination of EMG and Raman spectroscopy, achieved using a single needle.
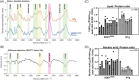
Methods: An optical EMG needle was created to collect electrophysiological and Raman spectroscopic data during a single insertion. We tested functionality with in vivo recordings in the SOD1G93A mouse model of amyotrophic lateral sclerosis (ALS), using both transgenic (n = 10) and non-transgenic (NTg, n = 7) mice. Under anesthesia, compound muscle action potentials (CMAPs), spontaneous EMG activity and Raman spectra were recorded from both gastrocnemius muscles with the optical EMG needle. Standard concentric EMG needle recordings were also undertaken. Electrophysiological data were analyzed with standard univariate statistics, Raman data with both univariate and multivariate analyses.
Results: A significant difference in CMAP amplitude was observed between SOD1G93A and NTg mice with optical EMG and standard concentric needles (p = .015 and p = .011, respectively). Spontaneous EMG activity (positive sharp waves) was detected in transgenic SOD1G93A mice only. Raman spectra demonstrated peaks associated with key muscle components. Significant differences in molecular composition between SOD1G93A and NTg muscle were identified through the Raman spectra.
Discussion: Optical EMG can provide standard electrophysiological data and molecular Raman data during a single needle insertion and represents a potential biomarker for neuromuscular disease.
Keywords: ALS; EMG; Raman spectroscopy; biomarker; muscle; optical EMG.
© 2023 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
J.J.P.A. and J.C.C.D. are the joint authors of a UK patent application concerning the described technology, filed September 2022, number 2214072.7.
Figures


References
-
- Trojaborg W. The concentric versus the monopolar needle electrode. The case for concentric needles. Muscle Nerve. 1998;21(12):1806‐1808. - PubMed
-
- Traynor D, Behl I, O'Dea D, et al. Raman spectral cytopathology for cancer diagnostic applications. Nat Protoc. 2021;16(7):3716‐3735. - PubMed
-
- Gautam R, Vanga S, Madan A, Gayathri N, Nongthomba U, Umapathy S. Raman spectroscopic studies on screening of myopathies. Anal Chem. 2015;87(4):2187‐2194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

