Good manufacturing practice production of CD34+ progenitor-derived NK cells for adoptive immunotherapy in acute myeloid leukemia
- PMID: 37477653
- PMCID: PMC10491545
- DOI: 10.1007/s00262-023-03492-6
Good manufacturing practice production of CD34+ progenitor-derived NK cells for adoptive immunotherapy in acute myeloid leukemia
Abstract
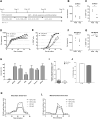
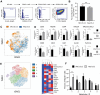
Allogeneic natural killer (NK) cell-based immunotherapy is a promising, well-tolerated adjuvant therapeutic approach for acute myeloid leukemia (AML). For reproducible NK cell immunotherapy, a homogenous, pure and scalable NK cell product is preferred. Therefore, we developed a good manufacturing practice (GMP)-compliant, cytokine-based ex vivo manufacturing process for generating NK cells from CD34+ hematopoietic stem and progenitor cells (HSPC). This manufacturing process combines amongst others IL15 and IL12 and the aryl hydrocarbon receptor antagonist StemRegenin-1 (SR1) to generate a consistent and active NK cell product that fits the requirements for NK cell immunotherapy well. The cell culture protocol was first optimized to generate NK cells with required expansion and differentiation capacity in GMP-compliant closed system cell culture bags. In addition, phenotype, antitumor potency, proliferative and metabolic capacity were evaluated to characterize the HSPC-NK product. Subsequently, seven batches were manufactured for qualification of the process. All seven runs demonstrated consistent results for proliferation, differentiation and antitumor potency, and preliminary specifications for the investigational medicinal product for early clinical phase trials were set. This GMP-compliant manufacturing process for HSPC-NK cells (named RNK001 cells) is used to produce NK cell batches applied in the clinical trial 'Infusion of ex vivo-generated allogeneic natural killer cells in combination with subcutaneous IL2 in patients with acute myeloid leukemia' approved by the Dutch Ethics Committee (EudraCT 2019-001929-27).
Keywords: Adoptive transfer; Cell manufacturing; Good manufacturing practice (GMP); Immunotherapy; Natural killer (NK) cells.
© 2023. The Author(s).
Conflict of interest statement
This work was supported by the Dutch Cancer Society KWF, grant numbers 10100 and 11564, and NIH grants R01CA205239, P50CA171963. Radboudumc is patent holder of the improved method for ex vivo expansion CD34+ HSPCs into NK cells using an aryl hydrocarbon receptor antagonist (PCT/EP2016/071660). The authors have no other relevant financial or non-financial interests to disclose.
Figures


References
-
- Shallis RM et al (2019) Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev 36:70–87 - PubMed
-
- Anthias C et al (2014) Pre-transplant MRD predicts outcome following reduced-intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transplant 49(5):679–683 - PubMed
-
- Curti A et al (2011) Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 118(12):3273–3279 - PubMed
-
- Miller JS et al (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105(8):3051–3057 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

