Immunologic and Genetic Contributors to CD46-Dependent Immune Dysregulation
- PMID: 37477760
- PMCID: PMC10661731
- DOI: 10.1007/s10875-023-01547-y
Immunologic and Genetic Contributors to CD46-Dependent Immune Dysregulation
Erratum in
-
Correction to: Immunologic and Genetic Contributors to CD46‑Dependent Immune Dysregulation.J Clin Immunol. 2023 Nov;43(8):1857. doi: 10.1007/s10875-023-01563-y. J Clin Immunol. 2023. PMID: 37572200 Free PMC article. No abstract available.
Abstract
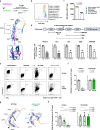
Mutations in CD46 predispose to atypical hemolytic uremic syndrome (aHUS) with low penetrance. Factors driving immune-dysregulatory disease in individual mutation carriers have remained ill-understood. In addition to its role as a negative regulator of the complement system, CD46 modifies T cell-intrinsic metabolic adaptation and cytokine production. Comparative immunologic analysis of diseased vs. healthy CD46 mutation carriers has not been performed in detail yet. In this study, we comprehensively analyzed clinical, molecular, immune-phenotypic, cytokine secretion, immune-metabolic, and genetic profiles in healthy vs. diseased individuals carrying a rare, heterozygous CD46 mutation identified within a large single family. Five out of six studied individuals carried a CD46 gene splice-site mutation causing an in-frame deletion of 21 base pairs. One child suffered from aHUS and his paternal uncle manifested with adult-onset systemic lupus erythematosus (SLE). Three mutation carriers had no clinical evidence of CD46-related disease to date. CD4+ T cell-intrinsic CD46 expression was uniformly 50%-reduced but was comparable in diseased vs. healthy mutation carriers. Reconstitution experiments defined the 21-base pair-deleted CD46 variant as intracellularly-but not surface-expressed and haploinsufficient. Both healthy and diseased mutation carriers displayed reduced CD46-dependent T cell mitochondrial adaptation. Diseased mutation carriers had lower peripheral regulatory T cell (Treg) frequencies and carried potentially epistatic, private rare variants in other inborn errors of immunity (IEI)-associated proinflammatory genes, not found in healthy mutation carriers. In conclusion, low Treg and rare non-CD46 immune-gene variants may contribute to clinically manifest CD46 haploinsufficiency-associated immune-dysregulation.
Keywords: CD46; Inborn errors of immunity; SLE; aHUS; atypical hemolytic uremic syndrome; haploinsufficiency; next-generation sequencing; penetrance; primary immunodeficiency complement; systemic lupus erythematosus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Yamamoto H, Fara AF, Dasgupta P, Kemper C. CD46: the 'multitasker' of complement proteins. Int J Biochem Cell Biol. 2013;45(12):2808–2820. - PubMed
-
- Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. - PubMed
-
- Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7(1):9–18. - PubMed
-
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421(6921):388–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

