A general overview of the multifactorial adaptation to cold: biochemical mechanisms and strategies
- PMID: 37477802
- PMCID: PMC10484896
- DOI: 10.1007/s42770-023-01057-4
A general overview of the multifactorial adaptation to cold: biochemical mechanisms and strategies
Abstract
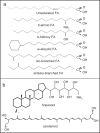
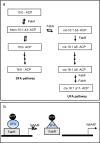
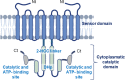
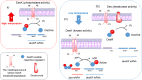
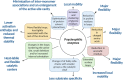
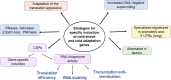
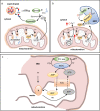
Cold environments are more frequent than people think. They include deep oceans, cold lakes, snow, permafrost, sea ice, glaciers, cold soils, cold deserts, caves, areas at elevations greater than 3000 m, and also artificial refrigeration systems. These environments are inhabited by a diversity of eukaryotic and prokaryotic organisms that must adapt to the hard conditions imposed by cold. This adaptation is multifactorial and includes (i) sensing the cold, mainly through the modification of the liquid-crystalline membrane state, leading to the activation of a two-component system that transduce the signal; (ii) adapting the composition of membranes for proper functions mainly due to the production of double bonds in lipids, changes in hopanoid composition, and the inclusion of pigments; (iii) producing cold-adapted proteins, some of which show modifications in the composition of amino acids involved in stabilizing interactions and structural adaptations, e.g., enzymes with high catalytic efficiency; and (iv) producing ice-binding proteins and anti-freeze proteins, extracellular polysaccharides and compatible solutes that protect cells from intracellular and extracellular ice. However, organisms also respond by reprogramming their metabolism and specifically inducing cold-shock and cold-adaptation genes through strategies such as DNA supercoiling, distinctive signatures in promoter regions and/or the action of CSPs on mRNAs, among others. In this review, we describe the main findings about how organisms adapt to cold, with a focus in prokaryotes and linking the information with findings in eukaryotes.
Keywords: Cold adaptation; Cold-shock proteins; Compatible solutes; Metabolism; Psychrophiles.
© 2023. The Author(s) under exclusive licence to Sociedade Brasileira de Microbiologia.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Helmke E, Weyland H. Psychrophilic versus psychrotolerant bacteria--occurrence and significance in polar and temperate marine habitats. Cell Mol Biol (Noisy-le-grand) 2004;50(5):553–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

