Fatty acid desaturase 2 determines the lipidomic landscape and steroidogenic function of the adrenal gland
- PMID: 37478183
- PMCID: PMC10361602
- DOI: 10.1126/sciadv.adf6710
Fatty acid desaturase 2 determines the lipidomic landscape and steroidogenic function of the adrenal gland
Abstract
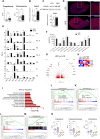
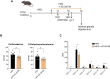
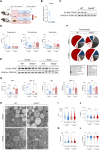
Corticosteroids regulate vital processes, including stress responses, systemic metabolism, and blood pressure. Here, we show that corticosteroid synthesis is related to the polyunsaturated fatty acid (PUFA) content of mitochondrial phospholipids in adrenocortical cells. Inhibition of the rate-limiting enzyme of PUFA synthesis, fatty acid desaturase 2 (FADS2), leads to perturbations in the mitochondrial lipidome and diminishes steroidogenesis. Consistently, the adrenocortical mitochondria of Fads2-/- mice fed a diet with low PUFA concentration are structurally impaired and corticoid levels are decreased. On the contrary, FADS2 expression is elevated in the adrenal cortex of obese mice, and plasma corticosterone is increased, which can be counteracted by dietary supplementation with the FADS2 inhibitor SC-26192 or icosapent ethyl, an eicosapentaenoic acid ethyl ester. In humans, FADS2 expression is elevated in aldosterone-producing adenomas compared to non-active adenomas or nontumorous adrenocortical tissue and correlates with expression of steroidogenic genes. Our data demonstrate that FADS2-mediated PUFA synthesis determines adrenocortical steroidogenesis in health and disease.
Figures




References
-
- R. D. Feldman, Aldosterone and blood pressure Regulation. Hypertension 63, 19–21 (2014). - PubMed
-
- C. A. Koch, Cushing syndrome and glucocorticoid excess, in Disorders of Blood Pressure Regulation: Phenotypes, mechanisms, therapeutic options, A. Berbari, G. Mancia, Eds, (Springer, 2018), pp. 481–512.
-
- M. M. Swierczynska, I. Mateska, M. Peitzsch, S. R. Bornstein, T. Chavakis, G. Eisenhofer, V. Lamounier-Zepter, S. Eaton, Changes in morphology and function of adrenal cortex in mice fed a high-fat diet. Int. J. Obes. (Lond) 39, 321–330 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

