Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer
- PMID: 37478190
- PMCID: PMC10361597
- DOI: 10.1126/sciadv.adf7195
Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer
Abstract
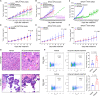
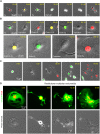
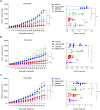
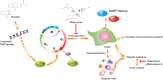
To understand the mechanism of acquired resistance to poly(ADP-ribose) polymerase inhibitors (PARPi) olaparib, we induced the formation of polyploid giant cancer cells (PGCCs) in ovarian and breast cancer cell lines, high-grade serous cancer (HGSC)-derived organoids, and patient-derived xenografts (PDXs). Time-lapse tracking of ovarian cancer cells revealed that PGCCs primarily developed from endoreplication after exposure to sublethal concentrations of olaparib. PGCCs exhibited features of senescent cells but, after olaparib withdrawal, can escape senescence via restitutional multipolar endomitosis and other noncanonical modes of cell division to generate mitotically competent resistant daughter cells. The contraceptive drug mifepristone blocked PGCC formation and daughter cell formation. Mifepristone/olaparib combination therapy substantially reduced tumor growth in PDX models without previous olaparib exposure, while mifepristone alone decreased tumor growth in PDX models with acquired olaparib resistance. Thus, targeting PGCCs may represent a promising approach to potentiate the therapeutic response to PARPi and overcome PARPi-induced resistance.
Figures




References
-
- H. E. Bryant, N. Schultz, H. D. Thomas, K. M. Parker, D. Flower, E. Lopez, S. Kyle, M. Meuth, N. J. Curtin, T. Helleday, Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005). - PubMed
-
- M. P. Dias, S. C. Moser, S. Ganesan, J. Jonkers, Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 18, 773–791 (2021). - PubMed
-
- R. L. Coleman, A. M. Oza, D. Lorusso, C. Aghajanian, A. Oaknin, A. Dean, N. Colombo, J. I. Weberpals, A. Clamp, G. Scambia, A. Leary, R. W. Holloway, M. A. Gancedo, P. C. Fong, J. C. Goh, D. M. O'Malley, D. K. Armstrong, J. Garcia-Donas, E. M. Swisher, A. Floquet, G. E. Konecny, I. A. McNeish, C. L. Scott, T. Cameron, L. Maloney, J. Isaacson, S. Goble, C. Grace, T. C. Harding, M. Raponi, J. Sun, K. K. Lin, H. Giordano, J. A. Ledermann; ARIEL3 investigators , Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 1949–1961 (2017). - PMC - PubMed
-
- K. A. Gelmon, M. Tischkowitz, H. Mackay, K. Swenerton, A. Robidoux, K. Tonkin, H. Hirte, D. Huntsman, M. Clemons, B. Gilks, R. Yerushalmi, E. Macpherson, J. Carmichael, A. Oza, Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 12, 852–861 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

