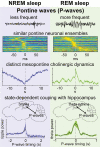
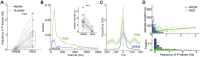
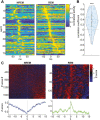
Pontine Waves Accompanied by Short Hippocampal Sharp Wave-Ripples During Non-rapid Eye Movement Sleep
- PMID: 37478470
- PMCID: PMC10485565
- DOI: 10.1093/sleep/zsad193
Pontine Waves Accompanied by Short Hippocampal Sharp Wave-Ripples During Non-rapid Eye Movement Sleep
Abstract
Ponto-geniculo-occipital or pontine (P) waves have long been recognized as an electrophysiological signature of rapid eye movement (REM) sleep. However, P-waves can be observed not just during REM sleep, but also during non-REM (NREM) sleep. Recent studies have uncovered that P-waves are functionally coupled with hippocampal sharp wave ripples (SWRs) during NREM sleep. However, it remains unclear to what extent P-waves during NREM sleep share their characteristics with P-waves during REM sleep and how the functional coupling to P-waves modulates SWRs. Here, we address these issues by performing multiple types of electrophysiological recordings and fiber photometry in both sexes of mice. P-waves during NREM sleep share their waveform shapes and local neural ensemble dynamics at a short (~100 milliseconds) timescale with their REM sleep counterparts. However, the dynamics of mesopontine cholinergic neurons are distinct at a longer (~10 seconds) timescale: although P-waves are accompanied by cholinergic transients, the cholinergic tone gradually reduces before P-wave genesis during NREM sleep. While P-waves are coupled to hippocampal theta rhythms during REM sleep, P-waves during NREM sleep are accompanied by a rapid reduction in hippocampal ripple power. SWRs coupled with P-waves are short-lived and hippocampal neural firing is also reduced after P-waves. These results demonstrate that P-waves are part of coordinated sleep-related activity by functionally coupling with hippocampal ensembles in a state-dependent manner.
Keywords: NREM sleep; PGO wave; REM sleep; sharp wave-ripples; theta rhythms.
© The Author(s) 2023. Published by Oxford University Press on behalf of Sleep Research Society.
Figures