Association between SARS-CoV-2 Symptoms, Ct Values, and Serological Response in Vaccinated and Unvaccinated Healthcare Personnel
- PMID: 37478837
- PMCID: PMC10482509
- DOI: 10.1093/jalm/jfad042
Association between SARS-CoV-2 Symptoms, Ct Values, and Serological Response in Vaccinated and Unvaccinated Healthcare Personnel
Abstract
Background: SARS-CoV-2 vaccines are effective at reducing symptomatic and asymptomatic COVID-19. Limited studies have compared symptoms, threshold cycle (Ct) values from reverse transcription (RT)-PCR testing, and serological testing results between previously vaccinated vs unvaccinated populations with SARS-CoV-2 infection.
Methods: Healthcare personnel (HCP) with a positive SARS-CoV-2 RT-PCR test within the previous 14 to 28 days completed surveys including questions about demographics, medical conditions, social factors, and symptoms of COVID-19. Ct values were observed, and serological testing was performed for anti-nucleocapsid (anti-N) and anti-Spike (anti-S) antibodies at enrollment and 40 to 90 days later. Serological results were compared to HCP with no known SARS-CoV-2 infection and negative anti-N testing.
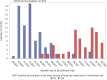
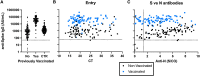
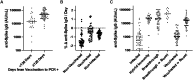
Results: There were 104 unvaccinated/not fully vaccinated and 77 vaccinated HCP with 2 doses of an mRNA vaccine at time of infection. No differences in type or duration of symptoms were reported (P = 0.45). The median (interquartile range [IQR]) Ct was 21.4 (17.6-24.6) and 21.5 (18.1-24.6) for the unvaccinated and vaccinated HCP, respectively. Higher anti-N IgG was observed in unvaccinated HCP (5.08 S/CO, 3.08-6.92) than vaccinated (3.61 signal to cutoff ratio [S/CO], 2.16-5.05). Anti-S IgG was highest among vaccinated HCP with infection (34 285 aribitrary units [AU]/mL, 17 672-61 775), followed by vaccinated HCP with no prior infection (1452 AU/mL, 791-2943), then unvaccinated HCP with infection (829 AU/mL, 290-1555). Anti-S IgG decreased 1.56% (0.9%-1.79%) per day in unvaccinated and 0.38% (0.03%-0.94%) in vaccinated HCP.
Conclusions: Vaccinated HCP infected with SARS-CoV-2 reported comparable symptoms and had similar Ct values relative to unvaccinated. However, vaccinated HCP had increased and prolonged anti-S and decreased anti-N response relative to unvaccinated.
© American Association for Clinical Chemistry 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

