The pathogenic "symphony" in type 1 diabetes: A disorder of the immune system, β cells, and exocrine pancreas
- PMID: 37478842
- PMCID: PMC10529265
- DOI: 10.1016/j.cmet.2023.06.018
The pathogenic "symphony" in type 1 diabetes: A disorder of the immune system, β cells, and exocrine pancreas
Abstract
Type 1 diabetes (T1D) is widely considered to result from the autoimmune destruction of insulin-producing β cells. This concept has been a central tenet for decades of attempts seeking to decipher the disorder's pathogenesis and prevent/reverse the disease. Recently, this and many other disease-related notions have come under increasing question, particularly given knowledge gained from analyses of human T1D pancreas. Perhaps most crucial are findings suggesting that a collective of cellular constituents-immune, endocrine, and exocrine in origin-mechanistically coalesce to facilitate T1D. This review considers these emerging concepts, from basic science to clinical research, and identifies several key remaining knowledge voids.
Keywords: autoimmunity; endocrinology; exocrine pancreas; immune cells; inflammation; insulin; insulitis; islet of Langerhans; type 1 diabetes.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

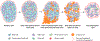
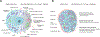

References
-
- Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark A, et al. (2015). Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38, 1964–1974. 10.2337/dc15-1419. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

