Influenza vaccine format mediates distinct cellular and antibody responses in human immune organoids
- PMID: 37478854
- PMCID: PMC10433940
- DOI: 10.1016/j.immuni.2023.06.019
Influenza vaccine format mediates distinct cellular and antibody responses in human immune organoids
Abstract
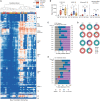
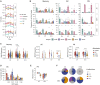
Highly effective vaccines elicit specific, robust, and durable adaptive immune responses. To advance informed vaccine design, it is critical that we understand the cellular dynamics underlying responses to different antigen formats. Here, we sought to understand how antigen-specific B and T cells were activated and participated in adaptive immune responses within the mucosal site. Using a human tonsil organoid model, we tracked the differentiation and kinetics of the adaptive immune response to influenza vaccine and virus modalities. Each antigen format elicited distinct B and T cell responses, including differences in their magnitude, diversity, phenotype, function, and breadth. These differences culminated in substantial changes in the corresponding antibody response. A major source of antigen format-related variability was the ability to recruit naive vs. memory B and T cells to the response. These findings have important implications for vaccine design and the generation of protective immune responses in the upper respiratory tract.
Keywords: B cells; B/T cell repertoire; T cells; adaptive immunity; antibodies; human immunology; influenza; organoids; vaccine modalities; vaccines.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.E.W. is the co-inventor of intellectual property assigned to Stanford University, “Systems and Methods to Model Adaptive Immune Responses,” which describes the immune organoid technology.
Figures





References
-
- Erbelding E.J., Post D.J., Stemmy E.J., Roberts P.C., Augustine A.D., Ferguson S., Paules C.I., Graham B.S., Fauci A.S. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018;218:347–354. doi: 10.1093/infdis/jiy103. - DOI - PMC - PubMed
-
- Nachbagauer R., Feser J., Naficy A., Bernstein D.I., Guptill J., Walter E.B., Berlanda-Scorza F., Stadlbauer D., Wilson P.C., Aydillo T., et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021;27:106–114. doi: 10.1038/s41591-020-1118-7. - DOI - PubMed
-
- Krammer F., Fouchier R.A.M., Eichelberger M.C., Webby R.J., Shaw-Saliba K., Wan H., Wilson P.C., Compans R.W., Skountzou I., Monto A.S. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio. 2018;9:e02332-17. doi: 10.1128/mBio.02332-17. - DOI - PMC - PubMed
-
- Park J., Fong S., Schwartzman L.M., Sheng Z.-M., Freeman A., Matthews L., Xiao Y., Ramuta M.D., Batchenkova N.A., Qi L., et al. An inactivated multivalent influenza A virus vaccine is broadly protective in mice and ferrets. Sci Transl. Med. 2021;14:eabo2167. doi: 10.1126/scitranslmed.abo2167. - DOI - PMC - PubMed

