Integrated NMR/Molecular Dynamics Determination of the Ensemble Conformation of a Thermodynamically Stable CUUG RNA Tetraloop
- PMID: 37479220
- PMCID: PMC10401711
- DOI: 10.1021/jacs.3c03578
Integrated NMR/Molecular Dynamics Determination of the Ensemble Conformation of a Thermodynamically Stable CUUG RNA Tetraloop
Abstract
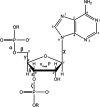
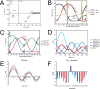
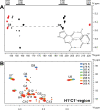
Both experimental and theoretical structure determinations of RNAs have remained challenging due to the intrinsic dynamics of RNAs. We report here an integrated nuclear magnetic resonance/molecular dynamics (NMR/MD) structure determination approach to describe the dynamic structure of the CUUG tetraloop. We show that the tetraloop undergoes substantial dynamics, leading to averaging of the experimental data. These dynamics are particularly linked to the temperature-dependent presence of a hydrogen bond within the tetraloop. Interpreting the NMR data by a single structure represents the low-temperature structure well but fails to capture all conformational states occurring at a higher temperature. We integrate MD simulations, starting from structures of CUUG tetraloops within the Protein Data Bank, with an extensive set of NMR data, and provide a structural ensemble that describes the dynamic nature of the tetraloop and the experimental NMR data well. We thus show that one of the most stable and frequently found RNA tetraloops displays substantial dynamics, warranting such an integrated structural approach.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




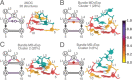
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

