Oxidation-State Dynamics and Emerging Patterns in Magnetite
- PMID: 37479223
- PMCID: PMC10405268
- DOI: 10.1021/acs.jpclett.3c01290
Oxidation-State Dynamics and Emerging Patterns in Magnetite
Abstract
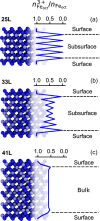
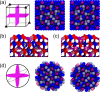
Magnetite is an important mineral with many interesting applications related to its magnetic, electrical, and thermal properties. Typically studied by electronic structure calculations, these methods are unable to capture the complex ion dynamics at relevant temperatures, time, and length scales. We present a hybrid Monte Carlo/molecular dynamics (MC/MD) method based on iron oxidation-state swapping for accurate atomistic modeling of bulk magnetite, magnetite surfaces, and nanoparticles that captures the complex ionic dynamics. By comparing the oxidation-state patterns with those obtained from density functional theory, we confirmed the accuracy of our approach. Lattice distortions leading to the stabilization of excess charges and a critical surface thickness at which the oxidation states transition from ordered to disordered were observed. This simple yet efficient approach paves the way for elucidating aspects of oxidation-state ordering of inverse spinel structures in general and battery materials in particular.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Bragg W. H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561–561. 10.1038/095561a0. - DOI
-
- Patitsa M.; Karathanou K.; Kanaki Z.; Tzioga L.; Pippa N.; Demetzos C.; Verganelakis D. A.; Cournia Z.; Klinakis A. Magnetic nanoparticles coated with polyarabic acid demonstrate enhanced drug delivery and imaging properties for cancer theranostic applications. Sci. Rep. 2017, 7, 775.10.1038/s41598-017-00836-y. - DOI - PMC - PubMed
-
- Zhang Q.; Yang X.; Guan J. Applications of magnetic nanomaterials in heterogeneous catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. 10.1021/acsanm.9b00976. - DOI
-
- Baeza A.; Guillena G.; Ramón D. J. Magnetite and Metal-Impregnated Magnetite Catalysts in Organic Synthesis: A Very Old Concept with New Promising Perspectives. ChemCatChem 2016, 8, 49–67. 10.1002/cctc.201500854. - DOI
LinkOut - more resources
Full Text Sources

