IgA antibody immunotherapy targeting GD2 is effective in preclinical neuroblastoma models
- PMID: 37479484
- PMCID: PMC10364159
- DOI: 10.1136/jitc-2023-006948
IgA antibody immunotherapy targeting GD2 is effective in preclinical neuroblastoma models
Abstract
Background: Immunotherapy targeting GD2 is very effective against high-risk neuroblastoma, though administration of anti-GD2 antibodies induces severe and dose-limiting neuropathic pain by binding GD2-expressing sensory neurons. Previously, the IgG1 ch14.18 (dinutuximab) antibody was reformatted into the IgA1 isotype, which abolishes neuropathic pain and induces efficient neutrophil-mediated antibody-dependent cellular cytotoxicity (ADCC) via activation of the Fc alpha receptor (FcαRI/CD89).
Methods: To generate an antibody suitable for clinical application, we engineered an IgA molecule (named IgA3.0 ch14.18) with increased stability, mutated glycosylation sites and substituted free (reactive) cysteines. The following mutations were introduced: N45.2G and P124R (CH1 domain), C92S, N120T, I121L and T122S (CH2 domain) and a deletion of the tail piece P131-Y148 (CH3 domain). IgA3.0 ch14.18 was evaluated in binding assays and in ADCC and antibody-dependent cellular phagocytosis (ADCP) assays with human, neuroblastoma patient and non-human primate effector cells. We performed mass spectrometry analysis of N-glycans and evaluated the impact of altered glycosylation in IgA3.0 ch14.18 on antibody half-life by performing pharmacokinetic (PK) studies in mice injected intravenously with 5 mg/kg antibody solution. A dose escalation study was performed to determine in vivo efficacy of IgA3.0 ch14.18 in an intraperitoneal mouse model using 9464D-GD2 neuroblastoma cells as well as in a subcutaneous human xenograft model using IMR32 neuroblastoma cells. Binding assays and PK studies were compared with one-way analysis of variance (ANOVA), ADCC and ADCP assays and in vivo tumor outgrowth with two-way ANOVA followed by Tukey's post-hoc test.
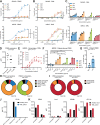
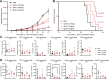
Results: ADCC and ADCP assays showed that particularly neutrophils and macrophages from healthy donors, non-human primates and patients with neuroblastoma are able to kill neuroblastoma tumor cells efficiently with IgA3.0 ch14.18. IgA3.0 ch14.18 contains a more favorable glycosylation pattern, corresponding to an increased antibody half-life in mice compared with IgA1 and IgA2. Furthermore, IgA3.0 ch14.18 penetrates neuroblastoma tumors in vivo and halts tumor outgrowth in both 9464D-GD2 and IMR32 long-term tumor models.
Conclusions: IgA3.0 ch14.18 is a promising new therapy for neuroblastoma, showing (1) increased half-life compared to natural IgA antibodies, (2) increased protein stability enabling effortless production and purification, (3) potent CD89-mediated tumor killing in vitro by healthy subjects and patients with neuroblastoma and (4) antitumor efficacy in long-term mouse neuroblastoma models.
Keywords: Antibody Glycosylation; Antibody Immunotherapy; GD-2; IgA; Neuroblastoma; Neutrophils; Tumor Immunology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JL and MdB are co-founder of TigaTx and JL is principal investigator on a research collaboration partially funded by TigaTx. ME, MN, ES, MJ and GvT were (partially) employed by TigaTx during the project and SK and RR are/were employed by Milabs. JL, GvT, ME are inventors on patent applications WO2019059771 and WO2020197400.
Figures





References
-
- Berthold F, Boos J, Burdach S, et al. . Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol 2005;6:649–58. 10.1016/S1470-2045(05)70291-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
