Elastin stabilization prevents impaired biomechanics in human pulmonary arteries and pulmonary hypertension in rats with left heart disease
- PMID: 37479718
- PMCID: PMC10362055
- DOI: 10.1038/s41467-023-39934-z
Elastin stabilization prevents impaired biomechanics in human pulmonary arteries and pulmonary hypertension in rats with left heart disease
Abstract
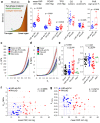
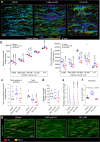
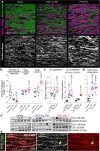
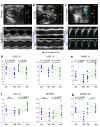
Pulmonary hypertension worsens outcome in left heart disease. Stiffening of the pulmonary artery may drive this pathology by increasing right ventricular dysfunction and lung vascular remodeling. Here we show increased stiffness of pulmonary arteries from patients with left heart disease that correlates with impaired pulmonary hemodynamics. Extracellular matrix remodeling in the pulmonary arterial wall, manifested by dysregulated genes implicated in elastin degradation, precedes the onset of pulmonary hypertension. The resulting degradation of elastic fibers is paralleled by an accumulation of fibrillar collagens. Pentagalloyl glucose preserves arterial elastic fibers from elastolysis, reduces inflammation and collagen accumulation, improves pulmonary artery biomechanics, and normalizes right ventricular and pulmonary hemodynamics in a rat model of pulmonary hypertension due to left heart disease. Thus, targeting extracellular matrix remodeling may present a therapeutic approach for pulmonary hypertension due to left heart disease.
© 2023. The Author(s).
Conflict of interest statement
N.R.V. holds significant equity in Elastrin Therapeutics Inc. which has licensed elastin-targeted nanoparticle therapy for cardiovascular and pulmonary conditions. V.F. receives remuneration, consultancy fees, and/or travel support outside the submitted work from Abbott GmbH & Co. KG, Novartis Pharma GmbH, Medtronic GmbH, Biotronic SE &Co., Boston Scientific, Edwards Lifesciences, Berlin Heart, JOTEC GmbH, Zurich Heart. M.M.K., W.M.K., and C.K. have filed a European Patent application (P30606WO) on “Pentagalloyl glucose (PGG) for use in the treatment of pulmonary hypertension”. The remaining authors declare no competing interests.
Figures



References
-
- Armentano RL, et al. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am. J. Physiol. 1991;260:H1870–H1877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

