Uropathogen and host responses in pyelonephritis
- PMID: 37479904
- PMCID: PMC10913074
- DOI: 10.1038/s41581-023-00737-6
Uropathogen and host responses in pyelonephritis
Abstract
Urinary tract infections (UTIs) are among the most common bacterial infections seen in clinical practice. The ascent of UTI-causing pathogens to the kidneys results in pyelonephritis, which can trigger kidney injury, scarring and ultimately impair kidney function. Despite sizable efforts to understand how infections develop or are cleared in the bladder, our appreciation of the mechanisms by which infections develop, progress or are eradicated in the kidney is limited. The identification of virulence factors that are produced by uropathogenic Escherichia coli to promote pyelonephritis have begun to fill this knowledge gap, as have insights into the mechanisms by which kidney tubular epithelial cells oppose uropathogenic E. coli infection to prevent or eradicate UTIs. Emerging data also illustrate how specific cellular immune responses eradicate infection whereas other immune cell populations promote kidney injury. Insights into the mechanisms by which uropathogenic E. coli circumvent host immune defences or antibiotic therapy to cause pyelonephritis is paramount to the development of new prevention and treatment strategies to mitigate pyelonephritis and its associated complications.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

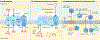
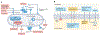
Similar articles
-
Neutrophil-Macrophage Imbalance Drives the Development of Renal Scarring during Experimental Pyelonephritis.J Am Soc Nephrol. 2021 Jan;32(1):69-85. doi: 10.1681/ASN.2020030362. Epub 2020 Nov 4. J Am Soc Nephrol. 2021. PMID: 33148615 Free PMC article.
-
Androgen exposure impairs neutrophil maturation and function within the infected kidney.mBio. 2024 Feb 14;15(2):e0317023. doi: 10.1128/mbio.03170-23. Epub 2024 Jan 11. mBio. 2024. PMID: 38206009 Free PMC article.
-
Genomic epidemiology and antibiotic susceptibility profiling of uropathogenic Escherichia coli among children in the United States.mSphere. 2023 Oct 24;8(5):e0018423. doi: 10.1128/msphere.00184-23. Epub 2023 Aug 15. mSphere. 2023. PMID: 37581436 Free PMC article.
-
Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli.Dan Med Bull. 2011 Apr;58(4):B4187. Dan Med Bull. 2011. PMID: 21466767 Review.
-
Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli.Mol Immunol. 2019 Apr;108:56-67. doi: 10.1016/j.molimm.2019.02.007. Epub 2019 Feb 18. Mol Immunol. 2019. PMID: 30784763 Review.
Cited by
-
DEFA1A3 DNA gene-dosage regulates the kidney innate immune response during upper urinary tract infection.Life Sci Alliance. 2024 Apr 5;7(6):e202302462. doi: 10.26508/lsa.202302462. Print 2024 Jun. Life Sci Alliance. 2024. PMID: 38580392 Free PMC article.
-
Current and emerging strategies to curb antibiotic-resistant urinary tract infections.Nat Rev Urol. 2024 Dec;21(12):707-722. doi: 10.1038/s41585-024-00877-9. Epub 2024 May 7. Nat Rev Urol. 2024. PMID: 38714857 Free PMC article. Review.
-
Analysing the influence of dapagliflozin on urinary tract infection vulnerability and kidney injury in mice infected with uropathogenic Escherichia coli.Diabetes Obes Metab. 2025 Jan;27(1):40-53. doi: 10.1111/dom.15981. Epub 2024 Sep 30. Diabetes Obes Metab. 2025. PMID: 39344841
-
Urinary tract infections: pathogenesis, host susceptibility and emerging therapeutics.Nat Rev Microbiol. 2025 Feb;23(2):72-86. doi: 10.1038/s41579-024-01092-4. Epub 2024 Sep 9. Nat Rev Microbiol. 2025. PMID: 39251839 Review.
-
The immune mechanisms of the urinary tract against infections.Front Cell Infect Microbiol. 2025 Apr 16;15:1540149. doi: 10.3389/fcimb.2025.1540149. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40308964 Free PMC article. Review.
References
-
- Becknell B, Schwaderer A, Hains DS & Spencer JD Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat. Rev. Nephrol. 11, 642–655 (2015). - PubMed
-
- Lacerda Mariano L & Ingersoll MA The immune response to infection in the bladder. Nat. Rev. Urol. 17, 439–458 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

