Comprehensive transcriptomic analysis of three varieties with different brown planthopper-resistance identifies leaf sheath lncRNAs in rice
- PMID: 37480003
- PMCID: PMC10362764
- DOI: 10.1186/s12870-023-04374-w
Comprehensive transcriptomic analysis of three varieties with different brown planthopper-resistance identifies leaf sheath lncRNAs in rice
Abstract
Background: Long non-coding RNAs (lncRNAs) have been brought great attention for their crucial roles in diverse biological processes. However, systematic identification of lncRNAs associated with specialized rice pest, brown planthopper (BPH), defense in rice remains unexplored.
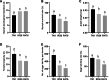
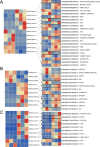
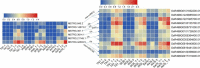
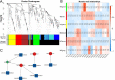
Results: In this study, a genome-wide high throughput sequencing analysis was performed using leaf sheaths of susceptible rice Taichung Native 1 (TN1) and resistant rice IR36 and R476 with and without BPH feeding. A total of 2283 lncRNAs were identified, of which 649 lncRNAs were differentially expressed. During BPH infestation, 84 (120 in total), 52 (70 in total) and 63 (94 in total) of differentially expressed lncRNAs were found only in TN1, IR36 and R476, respectively. Through analyzing their cis-, trans-, and target mimic-activities, not only the lncRNAs targeting resistance genes (NBS-LRR and RLKs) and transcription factors, but also the lncRNAs acting as the targets of the well-studied stress-related miRNAs (miR2118, miR528, and miR1320) in each variety were identified. Before the BPH feeding, 238 and 312 lncRNAs were found to be differentially expressed in TN1 vs. IR36 and TN1 vs. R476, respectively. Among their putative targets, the plant-pathogen interaction pathway was significantly enriched. It is speculated that the resistant rice was in a priming state by the regulation of lncRNAs. Furthermore, the lncRNAs extensively involved in response to BPH feeding were identified by Weighted Gene Co-expression Network Analysis (WGCNA), and the possible regulation networks of the key lncRNAs were constructed. These lncRNAs regulate different pathways that contribute to the basal defense and specific resistance of rice to the BPH.
Conclusion: In summary, we identified the specific lncRNAs targeting the well-studied stress-related miRNAs, resistance genes, and transcription factors in each variety during BPH infestation. Additionally, the possible regulating network of the lncRNAs extensively responding to BPH feeding revealed by WGCNA were constructed. These findings will provide further understanding of the regulatory roles of lncRNAs in BPH defense, and lay a foundation for functional research on the candidate lncRNAs.
Keywords: Expression analysis; Long non-coding RNAs; Nilaparvata lugens; Resistant rice; Transcriptome.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Comparative metabolomics analysis of different resistant rice varieties in response to the brown planthopper Nilaparvata lugens Hemiptera: Delphacidae.Metabolomics. 2019 Apr 11;15(4):62. doi: 10.1007/s11306-019-1523-4. Metabolomics. 2019. PMID: 30976994 Free PMC article.
-
Genome-wide identification of long non-coding (lncRNA) in Nilaparvata lugens's adaptability to resistant rice.PeerJ. 2022 Jul 25;10:e13587. doi: 10.7717/peerj.13587. eCollection 2022. PeerJ. 2022. PMID: 35910769 Free PMC article.
-
Gene expression and plant hormone levels in two contrasting rice genotypes responding to brown planthopper infestation.BMC Plant Biol. 2017 Feb 28;17(1):57. doi: 10.1186/s12870-017-1005-7. BMC Plant Biol. 2017. PMID: 28245796 Free PMC article.
-
Integrative Omics Strategies for Understanding and Combating Brown Planthopper Virulence in Rice Production: A Review.Int J Mol Sci. 2024 Oct 12;25(20):10981. doi: 10.3390/ijms252010981. Int J Mol Sci. 2024. PMID: 39456764 Free PMC article. Review.
-
Genetic and molecular understanding of host rice resistance and Nilaparvata lugens adaptation.Curr Opin Insect Sci. 2021 Jun;45:14-20. doi: 10.1016/j.cois.2020.11.005. Epub 2020 Nov 21. Curr Opin Insect Sci. 2021. PMID: 33227482 Review.
Cited by
-
Transcriptomic Comparison of Rice lncRNAs in Response to Feeding by Brown Planthopper Populations with Different Virulence.Int J Mol Sci. 2025 Apr 8;26(8):3486. doi: 10.3390/ijms26083486. Int J Mol Sci. 2025. PMID: 40331941 Free PMC article.
-
MicroRNA528 and Its Regulatory Roles in Monocotyledonous Plants.Int J Mol Sci. 2025 Jul 29;26(15):7334. doi: 10.3390/ijms26157334. Int J Mol Sci. 2025. PMID: 40806465 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2020A1515110063/Applied Basic Research Programs of Science and Technology Commission Foundation of Guangdong Province
- 2019A1515110867/Applied Basic Research Programs of Science and Technology Commission Foundation of Guangdong Province
- 32001903/National Natural Science Foundation of China
- 31901531/National Natural Science Foundation of China
- KA21031C5/Guangdong University Key Laboratory for Sustainable Control of Fruit and Vegetable Diseases and Pests
- KA21031C5/Guangdong University Key Laboratory for Sustainable Control of Fruit and Vegetable Diseases and Pests
- 2020A1515011052/Natural Science Foundation of Guangdong Province
- 2020A1515011052/Natural Science Foundation of Guangdong Province
- 2020ZDZX1013/Department of Education of Guangdong Province
- 2020ZDZX1013/Department of Education of Guangdong Province
- 2021A050530073/Department of Science and Technology of Guangdong Province
- 2021A050530073/Department of Science and Technology of Guangdong Province
- KB1708008/Agricultural and Rural Department of Guangdong Province
- KB1708008/Agricultural and Rural Department of Guangdong Province
- 202002010010/Guangzhou key laboratory for research and development of crop germplasm resources
- 202002010010/Guangzhou key laboratory for research and development of crop germplasm resources
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

