The role of PKN1 in glioma pathogenesis and the antiglioma effect of raloxifene targeting PKN1
- PMID: 37480215
- PMCID: PMC10494285
- DOI: 10.1111/jcmm.17860
The role of PKN1 in glioma pathogenesis and the antiglioma effect of raloxifene targeting PKN1
Abstract
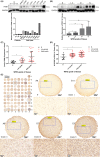
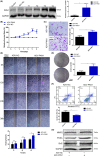
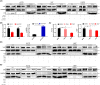
PKN1 (protein kinase N1), a serine/threonine protein kinase family member, is associated with various cancers. However, the role of PKN1 in gliomas has rarely been studied. We suggest that PKN1 expression in glioma specimens is considerably upregulated and positively correlates with the histopathological grading of gliomas. Knocking down PKN1 expression in glioblastoma (GBM) cells inhibits GBM cell proliferation, invasion and migration and promotes apoptosis. In addition, yes-associated protein (YAP) expression, an essential effector of the Hippo pathway contributing to the oncogenic role of gliomagenesis, was also downregulated. In contrast, PKN1 upregulation enhances the malignant characteristics of GBM cells and simultaneously upregulates YAP expression. Therefore, PKN1 is a promising therapeutic target for gliomas. Raloxifene (Ralo), a commonly used selective oestrogen-receptor modulator to treat osteoporosis in postmenopausal women, was predicted to target PKN1 according to the bioinformatics team from the School of Mathematics, Tianjin Nankai University. We showed that Ralo effectively targets PKN1, inhibits GBM cells proliferation and migration and sensitizes GBM cells to the major chemotherapeutic drug, Temozolomide. Ralo also reverses the effect of PKN1 on YAP activation. Thus, we confirm that PKN1 contributes to the pathogenesis of gliomas and may be a potential target for Ralo adjuvant glioma therapy.
Keywords: GBM; PKN1; TMZ; YAP; raloxifene.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





Similar articles
-
Apcin inhibits the growth and invasion of glioblastoma cells and improves glioma sensitivity to temozolomide.Bioengineered. 2021 Dec;12(2):10791-10798. doi: 10.1080/21655979.2021.2003927. Bioengineered. 2021. PMID: 34753395 Free PMC article.
-
Phosphorylated mTOR and YAP serve as prognostic markers and therapeutic targets in gliomas.Lab Invest. 2017 Nov;97(11):1354-1363. doi: 10.1038/labinvest.2017.70. Epub 2017 Jul 31. Lab Invest. 2017. PMID: 28759011
-
Intranasal Delivery of Temozolomide-Conjugated Gold Nanoparticles Functionalized with Anti-EphA3 for Glioblastoma Targeting.Mol Pharm. 2021 Mar 1;18(3):915-927. doi: 10.1021/acs.molpharmaceut.0c00911. Epub 2021 Jan 8. Mol Pharm. 2021. PMID: 33417456
-
Protein kinase N1 promotes proliferation and invasion of liver cancer.Exp Ther Med. 2021 Jun;21(6):651. doi: 10.3892/etm.2021.10083. Epub 2021 Apr 19. Exp Ther Med. 2021. PMID: 33968181 Free PMC article.
-
Serine/threonine/tyrosine-interacting-like protein 1 (STYXL1), a pseudo phosphatase, promotes oncogenesis in glioma.Biochem Biophys Res Commun. 2019 Jul 12;515(1):241-247. doi: 10.1016/j.bbrc.2019.05.093. Epub 2019 May 27. Biochem Biophys Res Commun. 2019. PMID: 31146910 Review.
Cited by
-
Epidermal stem cell-derived exosomes improve wound healing by promoting the proliferation and migration of human skin fibroblasts.Burns Trauma. 2024 Dec 16;12:tkae047. doi: 10.1093/burnst/tkae047. eCollection 2024. Burns Trauma. 2024. PMID: 39687464 Free PMC article.
-
NCOA4 inhibits glioma progression by suppressing the Sonic Hedgehog pathway and its overexpression indicates a better glioma prognosis.Genes Genomics. 2025 Aug 12. doi: 10.1007/s13258-025-01666-3. Online ahead of print. Genes Genomics. 2025. PMID: 40794264
-
POGZ targeted by LINC01355/miR-27b-3p retards thyroid cancer progression via interplaying with MAD2L2.3 Biotech. 2025 Apr;15(4):79. doi: 10.1007/s13205-025-04231-7. Epub 2025 Mar 8. 3 Biotech. 2025. PMID: 40071126
References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803‐820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

