Development of an eHealth-enhanced model of care for the monitoring and management of immune-related adverse events in patients treated with immune checkpoint inhibitors
- PMID: 37480546
- PMCID: PMC10363070
- DOI: 10.1007/s00520-023-07934-w
Development of an eHealth-enhanced model of care for the monitoring and management of immune-related adverse events in patients treated with immune checkpoint inhibitors
Abstract
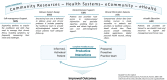
Purpose: The use of electronic patient-reported outcome (ePRO) data in routine care has been tied to direct patient benefits such as improved quality of care and symptom control and even overall survival. The modes of action behind such benefits are seldom described in detail. Here, we describe the development of a model of care leveraging ePRO data to monitor and manage symptoms of patients treated with immune checkpoint inhibitors.
Methods: Development was split into four stages: (1) identification of an underlying theoretical framework, (2) the selection of an ePRO measure (ePROM), (3) the adaptation of an electronic application to collect ePRO data, and (4) the description of an ePRO-oriented workflow. The model of care is currently evaluated in a bicentric longitudinal randomized controlled phase II trial, the IePRO study.
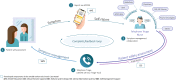
Results: The IePRO model of care is grounded in the eHealth Enhanced Chronic Care Model. Patients are prompted to report symptoms using an electronic mobile application. Triage nurses are alerted, review the reported symptoms, and contact patients in case of a new or worsening symptom. Nurses use the UKONS 24-hour telephone triage tool to issue patient management recommendations to the oncology team. Adapted care coordinating procedures facilitate team collaboration and provide patients with timely feedback.
Conclusion: This report clarifies how components of care are created and modified to leverage ePRO to enhance care. The model describes a workflow that enables care teams to be proactive and provide patients with timely, multidisciplinary support to manage symptoms.
Keywords: Immune-related adverse events; Model of care; Patient-reported outcomes; Remote symptom management; Self-management support.
© 2023. The Author(s).
Conflict of interest statement
A.D.S.L., C.D., S.G., S.B., G.G., V.A.L., N.M.A., S.L., and A. A. have no relevant financial or non-financial interests to disclose. S.C.L. reports grants from the ISREC Foundation. G.S-B. reports grants from MSD France, grants from Novartis, and personal fees from Bayer, MPNE, and WECAN, outside the submitted work. O.M. reports grants and personal fees from BMS, MSD, Pierre-Fabre, and Amgem; personal fees from Roche, Novartis, and GSL; and grants from Merck, outside the submitted work. M.E. received institutional research grants from Kaiku Health and reports grants from Bristol Myers Squibb, Roche and institutional fees as a Scientific Advisory Board Member/Consultant from Roche, outside the submitted work.
Figures

Similar articles
-
Testing a Model of Care for Patients on Immune Checkpoint Inhibitors Based on Electronic Patient-Reported Outcomes: Protocol for a Randomized Phase II Controlled Trial.JMIR Res Protoc. 2023 Oct 18;12:e48386. doi: 10.2196/48386. JMIR Res Protoc. 2023. PMID: 37851498 Free PMC article.
-
Co-design of an electronic patient-reported outcome symptom monitoring system for immunotherapy toxicities.Support Care Cancer. 2024 Dec 2;32(12):843. doi: 10.1007/s00520-024-09034-9. Support Care Cancer. 2024. PMID: 39623054
-
A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery.BMC Cancer. 2020 Jun 10;20(1):543. doi: 10.1186/s12885-020-07027-5. BMC Cancer. 2020. PMID: 32522163 Free PMC article.
-
Assessing Patient-Reported Outcomes in Routine Cancer Clinical Care Using Electronic Administration and Telehealth Technologies: Realist Synthesis of Potential Mechanisms for Improving Health Outcomes.J Med Internet Res. 2023 Nov 28;25:e48483. doi: 10.2196/48483. J Med Internet Res. 2023. PMID: 38015606 Free PMC article. Review.
-
Key methodological considerations for usability testing of electronic patient-reported outcome (ePRO) systems.Qual Life Res. 2020 Feb;29(2):325-333. doi: 10.1007/s11136-019-02329-z. Epub 2019 Oct 18. Qual Life Res. 2020. PMID: 31691202 Free PMC article. Review.
Cited by
-
Unleashing the potential of eHealth in outpatient cancer care for patients undergoing immunotherapy-a quantitative study considering patients' needs and current healthcare challenges.Front Digit Health. 2024 Oct 21;6:1414442. doi: 10.3389/fdgth.2024.1414442. eCollection 2024. Front Digit Health. 2024. PMID: 39498102 Free PMC article.
-
Cancer and treatment specific incidence rates of immune-related adverse events induced by immune checkpoint inhibitors: a systematic review.Br J Cancer. 2025 Jan;132(1):51-57. doi: 10.1038/s41416-024-02887-1. Epub 2024 Nov 3. Br J Cancer. 2025. PMID: 39489880 Free PMC article.
-
Testing a Model of Care for Patients on Immune Checkpoint Inhibitors Based on Electronic Patient-Reported Outcomes: Protocol for a Randomized Phase II Controlled Trial.JMIR Res Protoc. 2023 Oct 18;12:e48386. doi: 10.2196/48386. JMIR Res Protoc. 2023. PMID: 37851498 Free PMC article.
-
Co-design of an electronic patient-reported outcome symptom monitoring system for immunotherapy toxicities.Support Care Cancer. 2024 Dec 2;32(12):843. doi: 10.1007/s00520-024-09034-9. Support Care Cancer. 2024. PMID: 39623054
-
Wellbeing status and priority concerns of patients with advanced renal cell carcinoma: results of the EONS PROMs project international online survey.Support Care Cancer. 2025 Jun 3;33(6):531. doi: 10.1007/s00520-025-09585-5. Support Care Cancer. 2025. PMID: 40456970 Free PMC article.
References
-
- Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2017;28:iv119–42. 10.1093/annonc/mdx225 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

