The S1P receptor 1 antagonist Ponesimod reduces TLR4-induced neuroinflammation and increases Aβ clearance in 5XFAD mice
- PMID: 37480622
- PMCID: PMC10393615
- DOI: 10.1016/j.ebiom.2023.104713
The S1P receptor 1 antagonist Ponesimod reduces TLR4-induced neuroinflammation and increases Aβ clearance in 5XFAD mice
Abstract
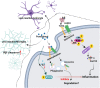
Background: Previously, we showed that the sphingosine-1-phosphate (S1P) transporter spinster 2 (Spns2) mediates activation of microglia in response to amyloid β peptide (Aβ). Here, we investigated if Ponesimod, a functional S1P receptor 1 (S1PR1) antagonist, prevents Aβ-induced activation of glial cells and Alzheimer's disease (AD) pathology.
Methods: We used primary cultures of glial cells and the 5XFAD mouse model to determine the effect of Aβ and Ponesimod on glial activation, Aβ phagocytosis, cytokine levels and pro-inflammatory signaling pathways, AD pathology, and cognitive performance.
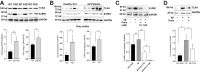
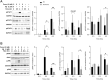
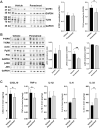
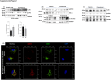
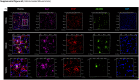
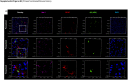
Findings: Aβ42 increased the levels of TLR4 and S1PR1, leading to their complex formation. Ponesimod prevented the increase in TLR4 and S1PR1 levels, as well as the formation of their complex. It also reduced the activation of the pro-inflammatory Stat1 and p38 MAPK signaling pathways, while activating the anti-inflammatory Stat6 pathway. This was consistent with increased phagocytosis of Aβ42 in primary cultured microglia. In 5XFAD mice, Ponesimod decreased the levels of TNF-α and CXCL10, which activate TLR4 and Stat1. It also increased the level of IL-33, an anti-inflammatory cytokine that promotes Aβ42 phagocytosis by microglia. As a result of these changes, Ponesimod decreased the number of Iba-1+ microglia and GFAP+ astrocytes, and the size and number of amyloid plaques, while improving spatial memory as measured in a Y-maze test.
Interpretation: Ponesimod targeting S1PR1 is a promising therapeutic approach to reprogram microglia, reduce neuroinflammation, and increase Aβ clearance in AD.
Funding: NIHR01AG064234, RF1AG078338, R21AG078601, VAI01BX003643.
Keywords: Alzheimer's disease; Neuroinflammation; Phagocytosis; Ponesimod; Sphingosine-1-phosphate; Toll-like receptor 4.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures











References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

