The genomic basis of temporal niche evolution in a diurnal rodent
- PMID: 37480852
- PMCID: PMC10529858
- DOI: 10.1016/j.cub.2023.06.068
The genomic basis of temporal niche evolution in a diurnal rodent
Abstract
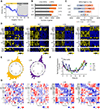
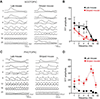
Patterns of diel activity-how animals allocate their activity throughout the 24-h daily cycle-play key roles in shaping the internal physiology of an animal and its relationship with the external environment.1,2,3,4,5 Although shifts in diel activity patterns have occurred numerous times over the course of vertebrate evolution,6 the genomic correlates of such transitions remain unknown. Here, we use the African striped mouse (Rhabdomys pumilio), a species that transitioned from the ancestrally nocturnal diel niche of its close relatives to a diurnal one,7,8,9,10,11 to define patterns of naturally occurring molecular variation in diel niche traits. First, to facilitate genomic analyses, we generate a chromosome-level genome assembly of the striped mouse. Next, using transcriptomics, we show that the switch to daytime activity in this species is associated with a realignment of daily rhythms in peripheral tissues with respect to the light:dark cycle and the central circadian clock. To uncover selection pressures associated with this temporal niche shift, we perform comparative genomic analyses with closely related rodent species and find evidence of relaxation of purifying selection on striped mouse genes in the rod phototransduction pathway. In agreement with this, electroretinogram measurements demonstrate that striped mice have functional differences in dim-light visual responses compared with nocturnal rodents. Taken together, our results show that striped mice have undergone a drastic change in circadian organization and provide evidence that the visual system has been a major target of selection as this species transitioned to a novel temporal niche.
Keywords: African striped mouse; Rhabdomys; circadian organization; circadian rhythm; comparative genomics; diurnality; molecular oscillator; phototransduction; temporal niche.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
Evolution: Out of the shadows and into the light.Curr Biol. 2023 Sep 25;33(18):R953-R955. doi: 10.1016/j.cub.2023.08.036. Curr Biol. 2023. PMID: 37751707
References
-
- Kronfeld-Schor N, and Dayan T (2003). Partitioning of Time as an Ecological Resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181.
-
- Penteriani V, del Mar Delgado M, Alonso-Alvarez C, and Sergio F (2006). The importance of visual cues for nocturnal species: eagle owls signal by badge brightness. Behav. Ecol. 18, 143–147.
-
- Schmitz L, and Motani R (2010). Morphological differences between the eyeballs of nocturnal and diurnal amniotes revisited from optical perspectives of visual environments. Vision Res. 50, 936–946. - PubMed
-
- Barton RA, Purvis A, and Harvey PH (1995). Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philos. Trans. R. Soc. Lond. B Biol. Sci. 348, 381–392. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

