Future medicine: from molecular pathways to the collective intelligence of the body
- PMID: 37481382
- PMCID: PMC10527237
- DOI: 10.1016/j.molmed.2023.06.007
Future medicine: from molecular pathways to the collective intelligence of the body
Abstract
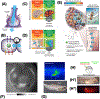
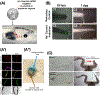
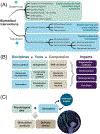
The remarkable anatomical homeostasis exhibited by complex living organisms suggests that they are inherently reprogrammable information-processing systems that offer numerous interfaces to their physiological and anatomical problem-solving capacities. We briefly review data suggesting that the multiscale competency of living forms affords a new path for biomedicine that exploits the innate collective intelligence of tissues and organs. The concept of tissue-level allostatic goal-directedness is already bearing fruit in clinical practice. We sketch a roadmap towards 'somatic psychiatry' by using advances in bioelectricity and behavioral neuroscience to design methods that induce self-repair of structure and function. Relaxing the assumption that cellular control mechanisms are static, exploiting powerful concepts from cybernetics, behavioral science, and developmental biology may spark definitive solutions to current biomedical challenges.
Keywords: bioelectricity; cognition; regenerative medicine; top-down control.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests M.L. is a cofounder of Morphoceuticals, a company working in the regenerative medicine space, and is supported by Astonishing Labs, a company that includes the targeting of computational capacities of pathways among its goals. E.L. is a cofounder of LyGenesis, a clinical stage cell therapy company that transforms a the lymph nodes of a patient into bioreactors that are able to grow functional ectopic organs.
Figures




References
-
- Baluska F et al. (2022) Cellular sentience as the primary source of biological order and evolution. Biosystems 218, 104694. - PubMed
-
- Baluska F et al. (2022) Cellular and evolutionary perspectives on organismal cognition: from unicellular to multicellular organisms. Biological Journal of the Linnean Society.
-
- Reber AS and Baluska F (2021) Cognition in some surprising places. Biochem Biophys Res Commun 564, 150–157. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

