Metabolism-guided development of Ko143 analogs as ABCG2 inhibitors
- PMID: 37482017
- PMCID: PMC10529637
- DOI: 10.1016/j.ejmech.2023.115666
Metabolism-guided development of Ko143 analogs as ABCG2 inhibitors
Abstract
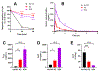
ATP-binding cassette subfamily G member 2 (ABCG2), an efflux transporter, is involved in multiple pathological processes. Ko143 is a potent ABCG2 inhibitor; however, it is quickly metabolized through carboxylesterase 1-mediated hydrolysis of its t-butyl ester moiety. The current work aimed to develop more metabolically stable ABCG2 inhibitors. Novel Ko143 analogs were designed and synthesized by replacing the unstable t-butyl ester moiety in Ko143 with an amide group. The synthesized Ko143 analogs were evaluated for their ABCG2 inhibitory activity, binding mode with ABCG2, cytotoxicity, and metabolic stability. We found that the amide modification of Ko143 led to metabolically stable ABCG2 inhibitors. Among these Ko143 analogs, K2 and K34 are promising candidates with favorable oral pharmacokinetic profiles in mice. In summary, we synthesized novel Ko143 analogs with improved metabolic stability, which can potentially be used as lead compounds for the future development of ABCG2 inhibitors.
Keywords: ABCG2 inhibitor; Carboxylesterase; Ko143 analogs; Metabolic stability; Pharmacokinetics.
Copyright © 2023 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest X.M., J.Z., and J.L. are inventors on a patent (WO2020236901) and hold equity in Portal Therapeutics, Inc. The authors declare no other competing interests.
Figures

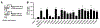

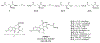
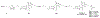
References
-
- Fletcher JI, Haber M, Henderson MJ, Norris MD, ABC transporters in cancer: more than just drug efflux pumps, Nat. Rev. Cancer, 10 (2010) 147–156. - PubMed
-
- Diestra JE, Scheffer GL, Catala I, Maliepaard M, Schellens JH, Scheper RJ, Germa-Lluch JR, Izquierdo MA, Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material, J Pathol, 198 (2002) 213–219. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

