Exploring diverse reactive warheads for the design of SARS-CoV-2 main protease inhibitors
- PMID: 37482021
- PMCID: PMC10529912
- DOI: 10.1016/j.ejmech.2023.115667
Exploring diverse reactive warheads for the design of SARS-CoV-2 main protease inhibitors
Abstract
SARS-CoV-2 main protease (Mpro) is a validated antiviral drug target of nirmatrelvir, the active ingredient in Pfizer's oral drug Paxlovid. Drug-drug interactions limit the use of Paxlovid. In addition, drug-resistant Mpro mutants against nirmatrelvir have been identified from cell culture viral passage and naturally occurring variants. As such, there is a need for a second generation of Mpro inhibitors. In this study, we explored several reactive warheads in the design of Mpro inhibitors. We identified Jun11119R (vinyl sulfonamide warhead), Jun10221R (propiolamide warhead), Jun1112R (4-chlorobut-2-ynamide warhead), Jun10541R (nitrile warhead), and Jun10963R (dually activated nitrile warhead) as potent Mpro inhibitors. Jun10541R and Jun10963R also had potent antiviral activity against SARS-CoV-2 in Calu-3 cells with EC50 values of 2.92 and 6.47 μM, respectively. X-ray crystal structures of Mpro with Jun10541R and Jun10221 revealed covalent modification of Cys145. These Mpro inhibitors with diverse reactive warheads collectively represent promising candidates for further development.
Keywords: 3CL protease; Antiviral; COVID-19; Main protease; SARS-CoV-2.
Copyright © 2023 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jun Wang reports financial support was provided by National Institute of Allergy and Infectious Diseases. Jun Wang has patent #WO2022119756A1 pending to University of Arizona.
Figures
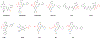
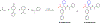

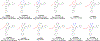
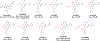

Similar articles
-
Recent Advances in SARS-CoV-2 Main Protease Inhibitors: From Nirmatrelvir to Future Perspectives.Biomolecules. 2023 Sep 2;13(9):1339. doi: 10.3390/biom13091339. Biomolecules. 2023. PMID: 37759739 Free PMC article. Review.
-
Silaproline-bearing nirmatrelvir derivatives are potent inhibitors of the SARS-CoV-2 main protease highlighting the value of silicon-derivatives in structure-activity-relationship studies.Eur J Med Chem. 2025 Jul 5;291:117603. doi: 10.1016/j.ejmech.2025.117603. Epub 2025 Apr 7. Eur J Med Chem. 2025. PMID: 40220677
-
SARS-CoV-2 Main Protease Drug Design, Assay Development, and Drug Resistance Studies.Acc Chem Res. 2023 Jan 17;56(2):157-168. doi: 10.1021/acs.accounts.2c00735. Epub 2022 Dec 29. Acc Chem Res. 2023. PMID: 36580641 Free PMC article.
-
Genetic Surveillance of SARS-CoV-2 Mpro Reveals High Sequence and Structural Conservation Prior to the Introduction of Protease Inhibitor Paxlovid.mBio. 2022 Aug 30;13(4):e0086922. doi: 10.1128/mbio.00869-22. Epub 2022 Jul 13. mBio. 2022. PMID: 35862764 Free PMC article.
-
[Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination].Rev Esp Quimioter. 2022 Jun;35(3):236-240. doi: 10.37201/req/002.2022. Epub 2022 Feb 21. Rev Esp Quimioter. 2022. PMID: 35183067 Free PMC article. Review. Spanish.
Cited by
-
Advances in the Search for SARS-CoV-2 Mpro and PLpro Inhibitors.Pathogens. 2024 Sep 24;13(10):825. doi: 10.3390/pathogens13100825. Pathogens. 2024. PMID: 39452697 Free PMC article. Review.
-
Recent Advances in SARS-CoV-2 Main Protease Inhibitors: From Nirmatrelvir to Future Perspectives.Biomolecules. 2023 Sep 2;13(9):1339. doi: 10.3390/biom13091339. Biomolecules. 2023. PMID: 37759739 Free PMC article. Review.
-
On the origins of SARS-CoV-2 main protease inhibitors.RSC Med Chem. 2023 Oct 13;15(1):81-118. doi: 10.1039/d3md00493g. eCollection 2024 Jan 25. RSC Med Chem. 2023. PMID: 38283212 Free PMC article. Review.
-
Structure-Based Lead Optimization of Enterovirus D68 2A Protease Inhibitors.J Med Chem. 2023 Nov 9;66(21):14544-14563. doi: 10.1021/acs.jmedchem.3c00995. Epub 2023 Oct 19. J Med Chem. 2023. PMID: 37857371 Free PMC article.
-
Design, Synthesis, X-ray Crystallography, and Biological Activities of Covalent, Non-Peptidic Inhibitors of SARS-CoV-2 Main Protease.ACS Infect Dis. 2024 Feb 9;10(2):715-731. doi: 10.1021/acsinfecdis.3c00565. Epub 2024 Jan 8. ACS Infect Dis. 2024. PMID: 38192109 Free PMC article.
References
-
- https://coronavirus.jhu.edu/map.html Accessed on July 15th, 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

