A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis
- PMID: 37482172
- PMCID: PMC10800684
- DOI: 10.1016/j.cgh.2023.07.008
A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis
Abstract
Background & aims: Cannabis (delta-9-tetrahydrocannabinol), a nonselective cannabinoid-receptor agonist, relieves nausea and pain. Cannabidiol (CBD), a cannabinoid receptor 2 inverse agonist with central effects, also reduces gut sensation and inflammation. We compared the effects of 4 weeks of treatment with pharmaceutical CBD vs placebo in patients with idiopathic or diabetic (diabetes mellitus) gastroparesis.
Methods: We performed a randomized, double-blinded, placebo-controlled study of CBD twice daily (Epidiolex escalated to 20 mg/kg/d; Jazz Pharmaceuticals, Dublin, Ireland) in patients with nonsurgical gastroparesis with delayed gastric emptying of solids (GES). Symptoms were assessed by the Gastroparesis Cardinal Symptom Index Daily Diary. After 4 weeks of treatment, we measured GES, gastric volumes, and Ensure (Abbott Laboratories, Abbott Park, IL) satiation test (1 kcal/mL, 30 mL/min) to assess volume to comfortable fullness and maximum tolerance. Patients underwent specific FAAH and CNR1 genotyping. Statistical analysis compared 2 treatments using analysis of variance including baseline measurements and body mass index as covariates.
Results: Among 44 patients (32 idiopathic, 6 diabetes mellitus type 1, and 6 diabetes mellitus type 2), 5 patients did not tolerate full-dose escalation; 3 withdrew before completing 4 weeks of treatment (2 placebo, 1 CBD); 95% completed 4 weeks of treatment and diaries. Compared with placebo, CBD reduced the total Gastroparesis Cardinal Symptom Index score (P = .008), inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall symptom severity (P = .034). Patients treated with CBD had a higher volume to comfortable fullness and maximum tolerance and slower GES. FAAH rs34420 genotype significantly impacted nutrient drink ingestion. The most common adverse events reported were diarrhea (14 patients), fatigue (8 patients), headache (8 patients), and nausea (7 patients).
Conclusions: CBD provides symptom relief in patients with gastroparesis and improves the tolerance of liquid nutrient intake, despite slowing of GES.
Clinicaltrials: gov NCT #03941288.
Trial registration: ClinicalTrials.gov NCT03941288.
Keywords: Accommodation; Cannabinoid; FAAH; Receptor; Satiation; Stomach.
Copyright © 2023 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

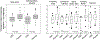
References
-
- Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SSC. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil 2008;20:635–642. - PubMed
-
- Ingrosso MR, Camilleri M, Tack J, Ianiro G, Black CJ, Ford AC. Efficacy and safety of drugs for gastroparesis: systematic review and network meta-analysis. Gastroenterology 2023;164:642–654. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

