A riboside hydrolase that salvages both nucleobases and nicotinamide in the auxotrophic parasite Trichomonas vaginalis
- PMID: 37482279
- PMCID: PMC10474468
- DOI: 10.1016/j.jbc.2023.105077
A riboside hydrolase that salvages both nucleobases and nicotinamide in the auxotrophic parasite Trichomonas vaginalis
Abstract
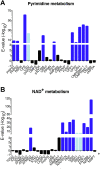
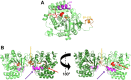
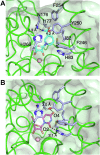
Pathogenic parasites of the Trichomonas genus are causative agents of sexually transmitted diseases affecting millions of individuals worldwide and whose outcome may include stillbirths and enhanced cancer risks and susceptibility to HIV infection. Trichomonas vaginalis relies on imported purine and pyrimidine nucleosides and nucleobases for survival, since it lacks the enzymatic activities necessary for de novo biosynthesis. Here we show that T. vaginalis additionally lacks homologues of the bacterial or mammalian enzymes required for the synthesis of the nicotinamide ring, a crucial component in the redox cofactors NAD+ and NADP. Moreover, we show that a yet fully uncharacterized T. vaginalis protein homologous to bacterial and protozoan nucleoside hydrolases is active as a pyrimidine nucleosidase but shows the highest specificity toward the NAD+ metabolite nicotinamide riboside. Crystal structures of the trichomonal riboside hydrolase in different states reveals novel intermediates along the nucleoside hydrolase-catalyzed hydrolytic reaction, including an unexpected asymmetry in the homotetrameric assembly. The active site structure explains the broad specificity toward different ribosides and offers precise insights for the engineering of specific inhibitors that may simultaneously target different essential pathways in the parasite.
Keywords: NAD; Trichomonas vaginalis; X-ray crystallography; drug design; enzyme structure; pyrimidine.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Dunne R.L., Dunn L.A., Upcroft P., O'Donoghue P.J., Upcroft J.A. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res. 2003;13:239–249. - PubMed
-
- Heyworth P.G., Gutteridge W.E., Ginger C.D. Purine metabolism in Trichomonas vaginalis. FEBS Lett. 1982;141:106–110. - PubMed
-
- Heyworth P.G., Gutteridge W.E., Ginger C.D. Pyrimidine metabolism in Trichomonas vaginalis. FEBS Lett. 1984;176:55–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

