Diversity-oriented synthesis of P-stereogenic and axially chiral monodentate biaryl phosphines enabled by C-P bond cleavage
- PMID: 37482556
- PMCID: PMC10363526
- DOI: 10.1038/s41467-023-40138-8
Diversity-oriented synthesis of P-stereogenic and axially chiral monodentate biaryl phosphines enabled by C-P bond cleavage
Abstract
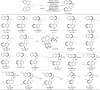
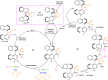
Chiral monodentate biaryl phosphines (MOPs) have attracted intense attention as chiral ligands over the past decades. However, the creation of structurally diverse chiral MOPs with both P- and axial chirality is still in high demand but challenging. Here, we show a distinct strategy for diversity-oriented synthesis of structurally diverse MOPs containing both P- and axial chirality enabled by enantioselective C-P bond cleavage. The key chiral PdII intermediates, generated through the stereoselective oxidative addition of C-P bond, could be trapped by alkynes, R3Si-Bpin, diboron esters or reduced by H2O/B2pin2, leading to enantioenriched structurally diverse MOPs in excellent diastereo- and enantioselectivities. Based on the outstanding properties of the parent scaffolds, the P- and axially chiral monodentate biaryl phosphines serve as excellent catalysts in asymmetric [3 + 2] annulation of MBH carbonate affording the chiral functionalized bicyclic imide.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Zhou, Q.-L. Privileged Chiral Ligands and Catalysts (Wiley-VCH, Weinheim, 2011).
-
- Kamer, P. C. J. & van Leeuwen, P. W. N. M. (eds) Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis (Wiley, Hoboken, 2012).
-
- Ni H, Chan W-L, Lu Y. Phosphine-catalyzed asymmetric organic reactions. Chem. Rev. 2018;118:9344–9411. - PubMed
-
- Tang W, Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003;103:3029–3069. - PubMed
-
- van Leeuwen PWNM, Kamer PCJ, Claver C, Pàmies O, Diéguez M. Phosphite-containing ligands for asymmetric catalysis. Chem. Rev. 2011;111:2077–2118. - PubMed
Grants and funding
- 22001008/National Natural Science Foundation of China (National Science Foundation of China)
- 22201009/National Natural Science Foundation of China (National Science Foundation of China)
- 92156022/National Natural Science Foundation of China (National Science Foundation of China)
- 1908085QB79/Natural Science Foundation of Anhui Province (Anhui Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Miscellaneous

