Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
- PMID: 37482654
- PMCID: PMC10695719
- DOI: 10.4093/dmj.2023.0072
Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
Abstract
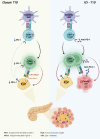
With the increasing use of immune-checkpoint inhibitors (ICIs), such as anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and anti-programmed cell death-1 (PD-1), for the treatment of malignancies, cases of ICI-induced type 1 diabetes mellitus (ICI-T1DM) have been reported globally. This review focuses on the features and pathogenesis of this disease. T1DM is an immune-related adverse event that occurs following the administration of anti-PD-1 or anti-programmed death ligand-1 (PDL1) alone or in combination with anti-CTLA-4. More than half of the reported cases presented as abrupt-onset diabetic ketoacidosis. The primary mechanism of ICI-T1DM is T-cell stimulation, which results from the loss of interaction between PD-1 and PD-L1 in pancreatic islet. The similarities and differences between ICI-T1DM and classical T1DM may provide insights into this disease entity. ICI-T1DM is a rare but often life-threatening medical emergency that healthcare professionals and patients need to be aware of. Early detection of and screening for this disease is imperative. At present, the only known treatment for ICI-T1DM is insulin injection. Further research into the mechanisms and risk factors associated with ICI-T1DM development may contribute to a better understanding of this disease entity and the identification of possible preventive strategies.
Keywords: Diabetes mellitus, type 1; Immune checkpoint inhibitors; Neoplasms.
Conflict of interest statement
Chang Hee Jung has been associate editor of the
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

